Identification of Glycycometus malaysiensis (for the first time in Brazil), Blomia tropicalis and Dermatophagoides pteronyssinus through multiplex PCR
- PMID: 35286553
- PMCID: PMC8919168
- DOI: 10.1007/s10493-022-00694-y
Identification of Glycycometus malaysiensis (for the first time in Brazil), Blomia tropicalis and Dermatophagoides pteronyssinus through multiplex PCR
Abstract
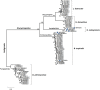
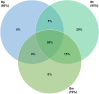
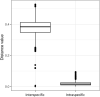
Blomia tropicalis and Dermatophagoides pteronyssinus play an important role in triggering allergy. Glycycometus malaysiensis causes IgE reaction in sensitive people, but is rarely reported in domestic dust, because it is morphologically similar to B. tropicalis making the identification of these species difficult. The identification of mites is mostly based on morphology, a time-consuming and ambiguous approach. Herein, we describe a multiplex polymerase chain reaction (mPCR) assay based on ribosomal DNA capable to identify mixed cultures of B. tropicalis, D. pteronyssinus and G. malaysiensis, and/or to identify these species from environmental dust. For this, the internal transcribed spacer 2 (ITS2) regions, flanked by partial sequences of the 5.8S and 28S genes, were PCR-amplified, cloned and sequenced. The sequences obtained were aligned with co-specific sequences available in the GenBank database for primer design and phylogenetic studies. Three pairs of primers were chosen to compose the mPCR assay, which was used to verify the frequency of different mites in house dust samples (n = 20) from homes of Salvador, Brazil. Blomia tropicalis was the most frequent, found in 95% of the samples, followed by G. malaysiensis (70%) and D. pteronyssinus (60%). Besides reporting for the first time the occurrence of G. malaysiensis in Brazil, our results confirm the good resolution of the ITS2 region for mite identification. Furthermore, the mPCR assay proved to be a fast and reliable tool for identifying these mites in mixed cultures and could be applied in future epidemiological studies, and for quality control of mite extract production for general use.
Keywords: Allergy; Blomia tropicalis; Dermatophagoides pteronyssinus; Glycycometus malaysiensis; Multiplex PCR; Species identification.
© 2022. The Author(s), under exclusive licence to Springer Nature Switzerland AG.
Conflict of interest statement
The authors declare that there is no conflict of interests regarding the publication of this paper.
Figures


References
-
- Alimuddin S, Rengganis I, Rumende CM, Setiati S. Comparison of specific immunoglobulin E with the skin prick test in the diagnosis of house dust mites and cockroach sensitization in patients with asthma and/or allergic rhinitis. Acta Med Indones. 2018;50:125–131. - PubMed
-
- Arlian LG, Berstein D, Berstein IL, Friedman S, Grant A, Lierbman P, Lopez M, Metzger J, Platts-Mills T, Schatz M. Prevalence of dust mites in the homes of people with asthma living in eight different geographic areas in United States. J Allergy Clin Immunol. 1992;90(3):292–300. doi: 10.1016/S0091-6749(05)80006-5. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

