Clinical Remission in Severe Asthma: A Pooled Post Hoc Analysis of the Patient Journey with Benralizumab
- PMID: 35287231
- PMCID: PMC9056458
- DOI: 10.1007/s12325-022-02098-1
Clinical Remission in Severe Asthma: A Pooled Post Hoc Analysis of the Patient Journey with Benralizumab
Abstract
Introduction: Consensus definitions for clinical remission and super-response were recently established for severe asthma. Benralizumab is an interleukin-5 (IL-5) receptor α-directed monoclonal antibody for severe, uncontrolled asthma; efficacy and safety were demonstrated in previous pivotal phase 3 trials (SIROCCO, CALIMA, ZONDA). This analysis applied a composite remission definition to characterize individual responses to benralizumab after 6 and 12 months.
Methods: In previous phase 3 studies, eligible patients were those with severe, uncontrolled asthma receiving medium- or high-dosage inhaled corticosteroids plus long-acting β2-agonists. This post hoc analysis included patients randomized to the approved benralizumab dose and not receiving oral corticosteroids (OCS) at baseline (SIROCCO/CALIMA) or OCS ≤ 12.5 mg per day (ZONDA). Individual remission components were zero exacerbations; zero OCS use; Asthma Control Questionnaire-6 (ACQ-6) score < 1.5 or ≤ 0.75; and pre-bronchodilator forced expiratory volume in 1 s (FEV1) increase ≥ 100 mL; clinical remission incorporated zero exacerbations, zero OCS use, ACQ-6 score ≤ 0.75, and pre-bronchodilator FEV1 increase ≥ 100 mL after 6 or 12 months.
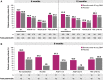
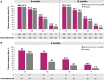
Results: Overall, 609 patients (N = 301 and N = 308) and 586 patients (N = 293 and N = 293) receiving benralizumab in SIROCCO and CALIMA were included at 6 and 12 months, respectively; 40 ZONDA patients were included after 6 months. In SIROCCO/CALIMA, similar to 6-month findings, approx. 83% and approx. 49% receiving benralizumab, and 77% and 37% on placebo achieved ≥ 2 and ≥ 3 remission components after 12 months; 14.5% (85/586) on benralizumab and 7.7% (48/620) on placebo achieved clinical remission at 12 months. Among ZONDA patients, 75% and approx. 48% on benralizumab and 35% and 20% on placebo achieved ≥ 2 and ≥ 3 remission components at 6 months, respectively; 22.5% (9/40) on benralizumab and 7.5% on placebo achieved clinical remission.
Conclusions: This analysis demonstrates clinical remission is achievable by targeting the underlying drivers of inflammation. Precision medicines can help shift treatment paradigms toward treat-to-target, with clinical remission as the ultimate therapeutic goal in severe asthma.
Clinical trial registration: SIROCCO (NCT01928771); CALIMA (NCT01914757); ZONDA (NCT02075255).
Keywords: Benralizumab; Biologic therapies; Oral corticosteroids (OCS); Precision medicine; Remission; Severe eosinophilic asthma; Super-response; Treat-to-target.
Plain language summary
Widely accepted definitions for disease remission are already established for the treatment of rheumatoid arthritis, ulcerative colitis, and cancer, among others. Two separate expert groups recently collaborated to discuss clinical remission/super-response to treatment in patients with severe asthma. Both groups developed separate, yet similar ways to determine whether a patient should be considered “in remission.” In this study, we used the results from three previous trials (SIROCCO, CALIMA, and ZONDA) that were conducted to assess a therapy called benralizumab in patients with severe asthma to identify patients who met some or all of the criteria for disease remission in severe asthma. These criteria included zero asthma exacerbations; zero oral steroid (OCS) use; asthma control score; and improvement in lung function. Across all three trials, about three quarters of the patients achieved two or more remission components and about half achieved three or more remission components after 6 months of treatment; furthermore, these rates were generally similar to the numbers of patients who achieved two or more components and three or more components of remission after 12 months of treatment. Overall, 15–23% of patients achieved clinical remission in 6 months, and approximately 15% achieved remission within 12 months. The results show that biologic therapies like benralizumab help improve the symptoms of severe asthma and allow patients to achieve disease remission.
© 2022. The Author(s).
Figures


Comment in
-
Letter to the Editor Regarding "Clinical Remission in Severe Asthma: A Pooled Post Hoc Analysis of the Patient Journey with Benralizumab".Adv Ther. 2022 Aug;39(8):3857-3861. doi: 10.1007/s12325-022-02213-2. Epub 2022 Jun 22. Adv Ther. 2022. PMID: 35731338 Free PMC article. No abstract available.
-
A Response to: Letter to the Editor Regarding "Clinical Remission in Severe Asthma: A Pooled Post Hoc Analysis of the Patient Journey with Benralizumab".Adv Ther. 2022 Aug;39(8):3862-3865. doi: 10.1007/s12325-022-02214-1. Epub 2022 Jun 22. Adv Ther. 2022. PMID: 35731339 Free PMC article. No abstract available.
References
-
- Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention, 2021 report. 2021 https://ginasthma.org/wp-content/uploads/2021/05/GINA-Main-Report-2021-V.... Accessed 1 Mar 2022.
-
- Global Burden of Disease and Injury Incidence and Prevalence Collaborators VT. Abajobir AA, Abbafati C, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet. 2017;390(10100):1211–1259. doi: 10.1016/S0140-6736(17)32154-2. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

