Safety and immunogenicity of a synthetic multiantigen modified vaccinia virus Ankara-based COVID-19 vaccine (COH04S1): an open-label and randomised, phase 1 trial
- PMID: 35287430
- PMCID: PMC8906816
- DOI: 10.1016/S2666-5247(22)00027-1
Safety and immunogenicity of a synthetic multiantigen modified vaccinia virus Ankara-based COVID-19 vaccine (COH04S1): an open-label and randomised, phase 1 trial
Abstract
Background: COH04S1, a synthetic attenuated modified vaccinia virus Ankara vector co-expressing SARS-CoV-2 spike and nucleocapsid antigens, was tested for safety and immunogenicity in healthy adults.
Methods: This combined open-label and randomised, phase 1 trial was done at the City of Hope Comprehensive Cancer Center (Duarte, CA, USA). We included participants aged 18-54 years with a negative SARS-CoV-2 antibody and PCR test, normal haematology and chemistry panels, a normal electrocardiogram and troponin concentration, negative pregnancy test if female, body-mass index of 30 kg/m2 or less, and no modified vaccinia virus Ankara or poxvirus vaccine in the past 12 months. In the open-label cohort, 1·0 × 107 plaque-forming units (PFU; low dose), 1·0 × 108 PFU (medium dose), and 2·5 × 108 PFU (high dose) of COH04S1 were administered by intramuscular injection on day 0 and 28 to sentinel participants using a queue-based statistical design to limit risk. In a randomised dose expansion cohort, additional participants were randomly assigned (3:3:1), using block size of seven, to receive two placebo vaccines (placebo group), one low-dose COH04S1 and one placebo vaccine (low-dose COH04S1 plus placebo group), or two low-dose COH04S1 vaccines (low-dose COH04S1 group). The primary outcome was safety and tolerability, with secondary objectives assessing vaccine-specific immunogenicity. The primary immunological outcome was a four times increase (seroconversion) from baseline in spike-specific or nucleocapsid-specific IgG titres within 28 days of the last injection, and seroconversion rates were compared with participants who received placebo using Fisher's exact test. Additional secondary outcomes included assessment of viral neutralisation and cellular responses. This trial is registered with ClinicalTrials.gov, NCT046339466.
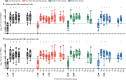
Findings: Between Dec 13, 2020, and May 24, 2021, 56 participants initiated vaccination. On day 0 and 28, 17 participants received low-dose COH04S1, eight received medium-dose COH04S1, nine received high-dose COH04S1, five received placebo, 13 received low-dose COH04S1 followed by placebo, and four discontinued early. Grade 3 fever was observed in one participant who received low-dose COH04S1 and placebo, and grade 2 anxiety or fatigue was seen in one participant who received medium-dose COH04S1. No severe adverse events were reported. Seroconversion was observed in all 34 participants for spike protein and 32 (94%) for nucleocapsid protein (p<0·0001 vs placebo for each comparison). Four times or more increase in SARS-CoV-2 neutralising antibodies within 56 days was measured in nine of 17 participants in the low-dose COH04S1 group, all eight participants in the medium-dose COH04S1 group, and eight of nine participants in the high-dose COH04S1 group (p=0·0035 combined dose levels vs placebo). Post-prime and post-boost four times increase in spike-specific or nucleocapsid-specific T cells secreting interferon-γ was measured in 48 (98%; 95% CI 89-100) of 49 participants who received at least one dose of COH04S1 and provided a sample for immunological analysis.
Interpretation: COH04S1 was well tolerated and induced spike-specific and nucleocapsid-specific antibody and T-cell responses. Future evaluation of this COVID-19 vaccine candidate as a primary or boost vaccination is warranted.
Funding: The Carol Moss Foundation and City of Hope Integrated Drug Development Venture programme.
© 2022 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license.
Conflict of interest statement
DJD and FW are co-inventors on a patent application covering the design and construction of the synthetic modified vaccinia Ankara platform (PCT/US2021/016247). DJD, FW, and FC are co-inventors on a patent application covering the development of a COVID-19 vaccine (PCT/US2021/032821). FC, JAZ, PHF, RS, JD, BW, AMA, KFr, RAT, JKD, SD, AGP, DDN, PA, YC, HC, CLR, KT, YP, JM, AI, QZ, VK, DJ, KFa, TK, JN, MK, VHN, SOF, AW, FW, and DJD are employees of City of Hope National Medical Center (Duarte, CA, USA), which developed the vaccine and funded the trial. AS is a consultant for Gritstone, Flow Pharma, Merck, Epitogenesis, Gilead, and Avalia. La Jolla Institute for Immunology has filed for patent protection for various aspects of T-cell epitope and vaccine design work. All other authors declare no competing interests.
Figures


References
-
- WHO WHO coronavirus (COVID-19) dashboard. https://covid19.who.int/
-
- Haas EJ, McLaughlin JM, Khan F, et al. Infections, hospitalisations, and deaths averted via a nationwide vaccination campaign using the Pfizer-BioNTech BNT162b2 mRNA COVID-19 vaccine in Israel: a retrospective surveillance study. Lancet Infect Dis. 2021 doi: 10.1016/S1473-3099(21)00566-1. published Sept 21. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

