TMEM106B deficiency impairs cerebellar myelination and synaptic integrity with Purkinje cell loss
- PMID: 35287730
- PMCID: PMC8919601
- DOI: 10.1186/s40478-022-01334-7
TMEM106B deficiency impairs cerebellar myelination and synaptic integrity with Purkinje cell loss
Abstract
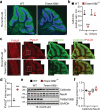
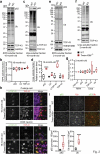
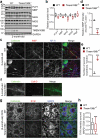
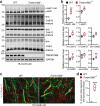
TMEM106B, a type II lysosomal transmembrane protein, has recently been associated with brain aging, hypomyelinating leukodystrophy, frontotemporal lobar degeneration (FTLD) and several other brain disorders. TMEM106B is critical for proper lysosomal function and TMEM106B deficiency leads to myelination defects, FTLD related pathology, and motor coordination deficits in mice. However, the physiological and pathological functions of TMEM106B in the brain are still not well understood. In this study, we investigate the role of TMEM106B in the cerebellum, dysfunction of which has been associated with FTLD and other brain disorders. We found that TMEM106B is ubiquitously expressed in neurons in the cerebellum, with the highest levels in the Purkinje neurons. Aged TMEM106B-deficient mice show significant loss of Purkinje neurons specifically in the anterior lobe of the cerebellum. Increased microglia and astrocyte activation, as well as an accumulation of ubiquitinated proteins, p62 and TDP-43 were also detected in the cerebellum of aged TMEM106B deficient mice. In the young mice, myelination defects and a significant loss of synapses between Purkinje and deep cerebellar nuclei neurons were observed. Interestingly, TMEM106B deficiency causes distinct lysosomal phenotypes in different types of neurons and glia in the cerebellum and frontal cortex. In humans, TMEM106B rs1990622 risk allele (T/T) is associated with increased Purkinje neuron loss. Taken together, our studies support that TMEM106B regulates lysosomal function in a cell-type-specific manner and TMEM106B is critical for maintaining synaptic integrity and neural functions in the cerebellum.
Keywords: Cerebellum; FTLD; Lysosome; Myelination; TMEM106B.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Elevated TMEM106B levels exaggerate lipofuscin accumulation and lysosomal dysfunction in aged mice with progranulin deficiency.Acta Neuropathol Commun. 2017 Jan 26;5(1):9. doi: 10.1186/s40478-017-0412-1. Acta Neuropathol Commun. 2017. PMID: 28126008 Free PMC article.
-
Physiological and pathological functions of TMEM106B: a gene associated with brain aging and multiple brain disorders.Acta Neuropathol. 2021 Mar;141(3):327-339. doi: 10.1007/s00401-020-02246-3. Epub 2021 Jan 1. Acta Neuropathol. 2021. PMID: 33386471 Free PMC article. Review.
-
Loss of TMEM106B and PGRN leads to severe lysosomal abnormalities and neurodegeneration in mice.EMBO Rep. 2020 Oct 5;21(10):e50219. doi: 10.15252/embr.202050219. Epub 2020 Aug 10. EMBO Rep. 2020. PMID: 32852886 Free PMC article.
-
Loss of TMEM106B exacerbates Tau pathology and neurodegeneration in PS19 mice.Acta Neuropathol. 2024 Mar 25;147(1):62. doi: 10.1007/s00401-024-02702-4. Acta Neuropathol. 2024. PMID: 38526799 Free PMC article.
-
What we know about TMEM106B in neurodegeneration.Acta Neuropathol. 2016 Nov;132(5):639-651. doi: 10.1007/s00401-016-1610-9. Epub 2016 Aug 20. Acta Neuropathol. 2016. PMID: 27543298 Free PMC article. Review.
Cited by
-
Flying under the radar: TMEM106B(120-254) fibrils break out in diverse neurodegenerative disorders.Cell. 2022 Apr 14;185(8):1290-1292. doi: 10.1016/j.cell.2022.03.032. Cell. 2022. PMID: 35427496 Free PMC article.
-
TMEM106B Knockdown Exhibits a Neuroprotective Effect in Parkinson's Disease via Decreasing Inflammation and Iron Deposition.Mol Neurobiol. 2025 Feb;62(2):1813-1825. doi: 10.1007/s12035-024-04373-4. Epub 2024 Jul 23. Mol Neurobiol. 2025. PMID: 39044012 Free PMC article.
-
Loss of TMEM106B exacerbates C9ALS/FTD DPR pathology by disrupting autophagosome maturation.Front Cell Neurosci. 2022 Dec 16;16:1061559. doi: 10.3389/fncel.2022.1061559. eCollection 2022. Front Cell Neurosci. 2022. PMID: 36619668 Free PMC article.
-
The role of endolysosomal progranulin and TMEM106B in neurodegenerative diseases.Mol Neurodegener. 2025 Jul 26;20(1):86. doi: 10.1186/s13024-025-00873-6. Mol Neurodegener. 2025. PMID: 40713630 Free PMC article. Review.
-
Multi-omic profiling reveals the ataxia protein sacsin is required for integrin trafficking and synaptic organization.Cell Rep. 2022 Nov 1;41(5):111580. doi: 10.1016/j.celrep.2022.111580. Cell Rep. 2022. PMID: 36323248 Free PMC article.
References
-
- Adamaszek M, D'Agata F, Ferrucci R, Habas C, Keulen S, Kirkby KC, Leggio M, Marien P, Molinari M, Moulton E, Orsi L, Van Overwalle F, Papadelis C, Priori A, Sacchetti B, Schutter DJ, Styliadis C, Verhoeven J. Consensus paper: cerebellum and emotion. Cerebellum. 2017;16:552–576. doi: 10.1007/s12311-016-0815-8. - DOI - PubMed
-
- Bharti K, Khan M, Beaulieu C, Graham SJ, Briemberg H, Frayne R, Genge A, Korngut L, Zinman L, Kalra S, Canadian ALSNC. Involvement of the dentate nucleus in the pathophysiology of amyotrophic lateral sclerosis: a multi-center and multi-modal neuroimaging study. Neuroimage Clin. 2020;28:102385. doi: 10.1016/j.nicl.2020.102385. - DOI - PMC - PubMed
-
- Bocchetta M, Todd EG, Peakman G, Cash DM, Convery RS, Russell LL, Thomas DL, Eugenio Iglesias J, van Swieten JC, Jiskoot LC, Seelaar H, Borroni B, Galimberti D, Sanchez-Valle R, Laforce R, Moreno F, Synofzik M, Graff C, Masellis M, Carmela Tartaglia M, Rowe JB, Vandenberghe R, Finger E, Tagliavini F, de Mendonca A, Santana I, Butler CR, Ducharme S, Gerhard A, Danek A, Levin J, Otto M, Sorbi S, Le Ber I, Pasquier F, Rohrer JD, Genetic Frontotemporal Dementia I Differential early subcortical involvement in genetic FTD within the GENFI cohort. Neuroimage Clin. 2021;30:102646. doi: 10.1016/j.nicl.2021.102646. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

