Seropositivity to Nucleoprotein to detect mild and asymptomatic SARS-CoV-2 infections: A complementary tool to detect breakthrough infections after COVID-19 vaccination?
- PMID: 35287986
- PMCID: PMC8904156
- DOI: 10.1016/j.vaccine.2022.03.009
Seropositivity to Nucleoprotein to detect mild and asymptomatic SARS-CoV-2 infections: A complementary tool to detect breakthrough infections after COVID-19 vaccination?
Abstract
Background: With COVID-19 vaccine roll-out ongoing in many countries globally, monitoring of breakthrough infections is of great importance. Antibodies persist in the blood after a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Since COVID-19 vaccines induce immune response to the Spike protein of the virus, which is the main serosurveillance target to date, alternative targets should be explored to distinguish infection from vaccination.
Methods: Multiplex immunoassay data from 1,513 SARS-CoV-2 RT-qPCR-tested individuals (352 positive and 1,161 negative) without COVID-19 vaccination history were used to determine the accuracy of Nucleoprotein-specific immunoglobulin G (IgG) in detecting past SARS-CoV-2 infection. We also described Spike S1 and Nucleoprotein-specific IgG responses in 230 COVID-19 vaccinated individuals (Pfizer/BioNTech).
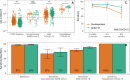
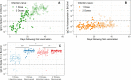
Results: The sensitivity of Nucleoprotein seropositivity was 85% (95% confidence interval: 80-90%) for mild COVID-19 in the first two months following symptom onset. Sensitivity was lower in asymptomatic individuals (67%, 50-81%). Participants who had experienced a SARS-CoV-2 infection up to 11 months preceding vaccination, as assessed by Spike S1 seropositivity or RT-qPCR, produced 2.7-fold higher median levels of IgG to Spike S1 ≥ 14 days after the first dose as compared to those unexposed to SARS-CoV-2 at ≥ 7 days after the second dose (p = 0.011). Nucleoprotein-specific IgG concentrations were not affected by vaccination in infection-naïve participants.
Conclusions: Serological responses to Nucleoprotein may prove helpful in identifying SARS-CoV-2 infections after vaccination. Furthermore, it can help interpret IgG to Spike S1 after COVID-19 vaccination as particularly high responses shortly after vaccination could be explained by prior exposure history.
Keywords: COVID-19; Multiplex immunoassay; Nucleoprotein; SARS-CoV-2; Serosurveillance; immunoglobulin G.
Copyright © 2022 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

