Pausing methotrexate improves immunogenicity of COVID-19 vaccination in elderly patients with rheumatic diseases
- PMID: 35288376
- PMCID: PMC9120396
- DOI: 10.1136/annrheumdis-2021-221876
Pausing methotrexate improves immunogenicity of COVID-19 vaccination in elderly patients with rheumatic diseases
Abstract
Objective: To study the effect of methotrexate (MTX) and its discontinuation on the humoral immune response after COVID-19 vaccination in patients with autoimmune rheumatic diseases (AIRD).
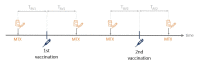
Methods: In this retrospective study, neutralising SARS-CoV-2 antibodies were measured after second vaccination in 64 patients with AIRD on MTX therapy, 31 of whom temporarily paused medication without a fixed regimen. The control group consisted of 21 patients with AIRD without immunosuppressive medication.
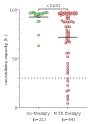
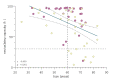
Results: Patients on MTX showed a significantly lower mean antibody response compared with patients with AIRD without immunosuppressive therapy (71.8% vs 92.4%, p<0.001). For patients taking MTX, age correlated negatively with immune response (r=-0.49; p<0.001). All nine patients with antibody levels below the cut-off were older than 60 years. Patients who held MTX during at least one vaccination showed significantly higher mean neutralising antibody levels after second vaccination, compared with patients who continued MTX therapy during both vaccinations (83.1% vs 61.2%, p=0.001). This effect was particularly pronounced in patients older than 60 years (80.8% vs 51.9%, p=0.001). The impact of the time period after vaccination was greater than of the time before vaccination with the critical cut-off being 10 days.
Conclusion: MTX reduces the immunogenicity of SARS-CoV-2 vaccination in an age-dependent manner. Our data further suggest that holding MTX for at least 10 days after vaccination significantly improves the antibody response in patients over 60 years of age.
Keywords: COVID-19; autoimmune diseases; methotrexate; vaccination.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: VMC is named together with Euroimmun GmbH on a patent application filed recently regarding the diagnostic of SARS-CoV-2 by antibody testing.
Figures

Comment in
-
Discontinuing methotrexate to enhance vaccine response.Nat Rev Rheumatol. 2022 Sep;18(9):497-498. doi: 10.1038/s41584-022-00817-0. Nat Rev Rheumatol. 2022. PMID: 35869395 Free PMC article.
References
-
- Ritchie H, Mathieu E, Rodés-Guirao L, et al. . Coronavirus pandemic (COVID-19): our world in data, 2020. Available: https://ourworldindata.org/coronavirus-data [Accessed 14 Nov 2021].
-
- Kane S. Methotrexate 2021. Available: https://clincalc.com/DrugStats/Drugs/Methotrexate [Accessed 14 Nov 2021].
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

