T cell receptor engineering of primary NK cells to therapeutically target tumors and tumor immune evasion
- PMID: 35288464
- PMCID: PMC8921915
- DOI: 10.1136/jitc-2021-003715
T cell receptor engineering of primary NK cells to therapeutically target tumors and tumor immune evasion
Abstract
Background: T cell receptor (TCR)-engineered cells can be powerful tools in the treatment of malignancies. However, tumor resistance by Human Leukocyte antigen (HLA) class I downregulation can negatively impact the success of any TCR-mediated cell therapy. Allogeneic natural killer (NK) cells have demonstrated efficacy and safety against malignancies without inducing graft-versus-host-disease, highlighting the feasibility for an 'off the shelf' cellular therapeutic. Furthermore, primary NK cells can target tumors using a broad array of intrinsic activation mechanisms. In this study, we combined the antitumor effector functions of NK cells with TCR engineering (NK-TCR), creating a novel therapeutic strategy to avoid TCR-associated immune resistance.
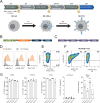
Methods: BOB1, is a transcription factor highly expressed in all healthy and malignant B cell lineages, including multiple myeloma (MM). Expression of an HLA-B*07:02 restricted BOB1-specifc TCR in peripheral blood-derived NK cells was achieved following a two-step retroviral transduction protocol. NK-TCR was then compared with TCR-negative NK cells and CD8-T cells expressing the same TCR for effector function against HLA-B*07:02+ B-cell derived lymphoblastoid cell lines (B-LCL), B-cell acute lymphoblastic leukemia and MM cell lines in vitro and in vivo.
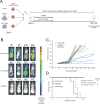
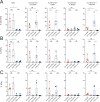
Results: Firstly, TCR could be reproducibly expressed in NK cells isolated from the peripheral blood of multiple healthy donors generating pure NK-TCR cell products. Secondly, NK-TCR demonstrated antigen-specific effector functions against malignancies which were previously resistant to NK-mediated lysis and enhanced NK efficacy in vivo using a preclinical xenograft model of MM. Moreover, antigen-specific cytotoxicity and cytokine production of NK-TCR was comparable to CD8 T cells expressing the same TCR. Finally, in a model of HLA-class I loss, tumor cells with B2M KO were lysed by NK-TCR in an NK-mediated manner but were resistant to T-cell based killing.
Conclusion: NK-TCR cell therapy enhances NK cell efficacy against tumors through additional TCR-mediated lysis. Furthermore, the dual efficacy of NK-TCR permits the specific targeting of tumors and the associated TCR-associated immune resistance, making NK-TCR a unique cellular therapeutic.
Keywords: cell engineering; immunologic; immunotherapy; killer cells; natural; receptors; tumor escape.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
