PI3Kδ/γ inhibitor BR101801 extrinsically potentiates effector CD8+ T cell-dependent antitumor immunity and abscopal effect after local irradiation
- PMID: 35288465
- PMCID: PMC8921929
- DOI: 10.1136/jitc-2021-003762
PI3Kδ/γ inhibitor BR101801 extrinsically potentiates effector CD8+ T cell-dependent antitumor immunity and abscopal effect after local irradiation
Abstract
Background: Radiotherapy enhances antitumor immunity. However, it also induces immunosuppressive responses, which are major hurdles for an effective treatment. Thus, targeting the immunosuppressive tumor microenvironment is essential for enhancing the antitumor immunity after radiotherapy. Retrospective studies show that a blockade of PI3Kδ and/or γ, which are abundant in leukocytes, exhibits antitumor immune response by attenuating activity of immune suppressive cells, however, the single blockade of PI3Kδ or γ is not sufficient to completely eliminate solid tumor.
Methods: We used BR101801, PI3Kδ/γ inhibitor in the CT-26 syngeneic mouse model with a subcutaneously implanted tumor. BR101801 was administered daily, and the target tumor site was locally irradiated. We monitored the tumor growth regularly and evaluated the immunological changes using flow cytometry, ELISpot, and transcriptional analysis.
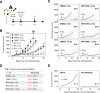
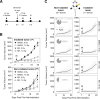
Results: This study showed that BR101801 combined with irradiation promotes systemic antitumor immunity and abscopal response by attenuating the activity of immune suppressive cells in the CT-26 tumor model. BR101801 combined with irradiation systemically reduced the proliferation of regulatory T cells (Tregs) and enhanced the number of tumor-specific CD8α+ T cells in the tumor microenvironment, thereby leading to tumor regression. Furthermore, the high ratio of CD8α+ T cells to Tregs was maintained for 14 days after irradiation, resulting in remote tumor regression in metastatic lesions, the so-called abscopal effect. Moreover, our transcriptomic analysis showed that BR101801 combined with irradiation promoted the immune-stimulatory tumor microenvironment, suggesting that the combined therapy converts immunologically cold tumors into hot one.
Conclusions: Our data suggest the first evidence that PI3Kδ/γ inhibition combined with irradiation promotes systemic antitumor immunity against solid tumors, providing the preclinical result of the potential use of PI3Kδ/γ inhibitor as an immune-regulatory radiosensitizer.
Keywords: immunotherapy; lymphocytes, tumor-infiltrating; radioimmunotherapy; radiotherapy.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
