DNA methylation-free Arabidopsis reveals crucial roles of DNA methylation in regulating gene expression and development
- PMID: 35288562
- PMCID: PMC8921224
- DOI: 10.1038/s41467-022-28940-2
DNA methylation-free Arabidopsis reveals crucial roles of DNA methylation in regulating gene expression and development
Abstract
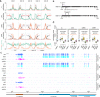
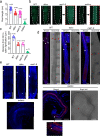
A contribution of DNA methylation to defense against invading nucleic acids and maintenance of genome integrity is uncontested; however, our understanding of the extent of involvement of this epigenetic mark in genome-wide gene regulation and plant developmental control is incomplete. Here, we knock out all five known DNA methyltransferases in Arabidopsis, generating DNA methylation-free plants. This quintuple mutant exhibits a suite of developmental defects, unequivocally demonstrating that DNA methylation is essential for multiple aspects of plant development. We show that CG methylation and non-CG methylation are required for a plethora of biological processes, including pavement cell shape, endoreduplication, cell death, flowering, trichome morphology, vasculature and meristem development, and root cell fate determination. Moreover, we find that DNA methylation has a strong dose-dependent effect on gene expression and repression of transposable elements. Taken together, our results demonstrate that DNA methylation is dispensable for Arabidopsis survival but essential for the proper regulation of multiple biological processes.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Zhang H, Lang Z, Zhu JK. Dynamics and function of DNA methylation in plants. Nat. Rev. Mol. Cell Biol. 2018;19:489–506. - PubMed
-
- Jullien PE, Susaki D, Yelagandula R, Higashiyama T, Berger F. DNA methylation dynamics during sexual reproduction in Arabidopsis thaliana. Curr. Biol. 2012;22:1825–1830. - PubMed
-
- Bond DM, Baulcombe DC. Small RNAs and heritable epigenetic variation in plants. Trends Cell Biol. 2014;24:100–107. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

