The MHC class I MICA gene is a histocompatibility antigen in kidney transplantation
- PMID: 35288692
- PMCID: PMC9117142
- DOI: 10.1038/s41591-022-01725-2
The MHC class I MICA gene is a histocompatibility antigen in kidney transplantation
Abstract
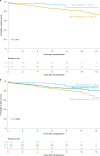
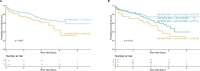
The identity of histocompatibility loci, besides human leukocyte antigen (HLA), remains elusive. The major histocompatibility complex (MHC) class I MICA gene is a candidate histocompatibility locus. Here, we investigate its role in a French multicenter cohort of 1,356 kidney transplants. MICA mismatches were associated with decreased graft survival (hazard ratio (HR), 2.12; 95% confidence interval (CI): 1.45-3.11; P < 0.001). Both before and after transplantation anti-MICA donor-specific antibodies (DSA) were strongly associated with increased antibody-mediated rejection (ABMR) (HR, 3.79; 95% CI: 1.94-7.39; P < 0.001; HR, 9.92; 95% CI: 7.43-13.20; P < 0.001, respectively). This effect was synergetic with that of anti-HLA DSA before and after transplantation (HR, 25.68; 95% CI: 3.31-199.41; P = 0.002; HR, 82.67; 95% CI: 33.67-202.97; P < 0.001, respectively). De novo-developed anti-MICA DSA were the most harmful because they were also associated with reduced graft survival (HR, 1.29; 95% CI: 1.05-1.58; P = 0.014). Finally, the damaging effect of anti-MICA DSA on graft survival was confirmed in an independent cohort of 168 patients with ABMR (HR, 1.71; 95% CI: 1.02-2.86; P = 0.041). In conclusion, assessment of MICA matching and immunization for the identification of patients at high risk for transplant rejection and loss is warranted.
© 2022. The Author(s).
Conflict of interest statement
D.A. has a patent ‘In vitro method for determining the likelihood of occurrence of an acute microvascular rejection (AMVR) against a renal allograft in an individual’ (EP19305037.4) issued. S.B. reports grants and personal fees from BIOMICA and personal fees from GenDx. S.C. reports non-financial support from Sanofi and Astellas, and non-financial support from Novartis, outside the submitted work. N.K. reports personal fees from Abbvie, Amgen, Astellas, Biotest, CSL Behring, Chiesi, Gilead, Fresenius Medical care, Merck Sharp and Dohme, Neovii, Novartis Pharma, Sanofi, Sandoz and Shire, outside the submitted work. P.P. reports personal fees from Chiesi, outside the submitted work. All other authors have no competing interests.
Figures



Comment in
-
MICA in kidney transplants.Nat Rev Nephrol. 2022 May;18(5):273. doi: 10.1038/s41581-022-00563-2. Nat Rev Nephrol. 2022. PMID: 35338343 No abstract available.
References
-
- Knechtle, S., Marson, L. & Morris, P. Kidney Transplantation – Principles and Practice (Elsevier, 2019).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

