Platelet-released extracellular vesicles: the effects of thrombin activation
- PMID: 35288766
- PMCID: PMC8920058
- DOI: 10.1007/s00018-022-04222-4
Platelet-released extracellular vesicles: the effects of thrombin activation
Abstract
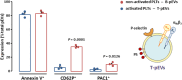
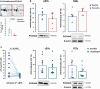
Platelets exert fundamental roles in thrombosis, inflammation, and angiogenesis, contributing to different pathologies from cardiovascular diseases to cancer. We previously reported that platelets release extracellular vesicles (pEVs) which contribute to thrombus formation. However, pEV composition remains poorly defined. Indeed, pEV quality and type, rather than quantity, may be relevant in intravascular cross-talk with either circulating or vascular cells. We aimed to define the phenotypic characteristics of pEVs released spontaneously and those induced by thrombin activation to better understand their role in disease dissemination. pEVs obtained from washed platelets from healthy donor blood were characterized by flow cytometry. pEVs from thrombin-activated platelets (T-pEVs) showed higher levels of P-selectin and active form of glycoprotein IIb/IIIa than baseline non-activated platelets (B-pEVs). Following mass spectrometry-based differential proteomic analysis, significant changes in the abundance of proteins secreted in T-pEVs compared to B-pEVs were found. These differential proteins were involved in coagulation, adhesion, cytoskeleton, signal transduction, metabolism, and vesicle-mediated transport. Interestingly, release of proteins relevant for cell adhesion, intrinsic pathway coagulation, and platelet activation signalling was significantly modified by thrombin stimulation. A novel pEV-associated protein (protocadherin-α4) was found to be significantly reduced in T-pEVs showing a shift towards increased expression in the membranes of activated platelets. In summary, platelet activation induced by thrombin triggers the shedding of pEVs with a complex proteomic pattern rich in procoagulant and proadhesive proteins. Crosstalk with other vascular and blood cells in a paracrine regulatory mode could extend the prothrombotic signalling as well as promote proteostasic changes in other cellular types.
Keywords: Atherosclerosis; Extracellular vesicles; Microvesicles; Platelets; Thrombin; Thrombosis.
© 2022. The Author(s), under exclusive licence to Springer Nature Switzerland AG.
Conflict of interest statement
R.S. has no relevant financial or non-financial interests to disclose. L.B. received a research grant from AstraZeneca; hold advisory board work for Sanofi, Bayer, and AstraZeneca; received speaker fees from Lilly, MSD-Boehringer, and AstraZeneca; and is founder and shareholder of Glycardial Diagnostics SL and Ivestatin Therapeutics SL (all outside of this work). G.V. and T.P. are founders and shareholders of Glycardial Diagnostics SL and Ivestatin Therapeutics (all outside of this work).
Figures





Similar articles
-
Platelet-derived extracellular vesicles released after trauma promote hemostasis and contribute to DVT in mice.J Thromb Haemost. 2019 Oct;17(10):1733-1745. doi: 10.1111/jth.14563. Epub 2019 Jul 28. J Thromb Haemost. 2019. PMID: 31294514 Free PMC article.
-
Platelet-derived- Extracellular Vesicles Promote Hemostasis and Prevent the Development of Hemorrhagic Shock.Sci Rep. 2019 Nov 27;9(1):17676. doi: 10.1038/s41598-019-53724-y. Sci Rep. 2019. PMID: 31776369 Free PMC article.
-
Dissecting the biochemical architecture and morphological release pathways of the human platelet extracellular vesiculome.Cell Mol Life Sci. 2018 Oct;75(20):3781-3801. doi: 10.1007/s00018-018-2771-6. Epub 2018 Feb 9. Cell Mol Life Sci. 2018. PMID: 29427073 Free PMC article.
-
Role of P2Y Receptors in Platelet Extracellular Vesicle Release.Int J Mol Sci. 2020 Aug 23;21(17):6065. doi: 10.3390/ijms21176065. Int J Mol Sci. 2020. PMID: 32842470 Free PMC article. Review.
-
Platelet and extracellular vesicles in COVID-19 infection and its vaccines.Transfus Apher Sci. 2022 Jun;61(3):103459. doi: 10.1016/j.transci.2022.103459. Epub 2022 May 21. Transfus Apher Sci. 2022. PMID: 35654711 Free PMC article. Review.
Cited by
-
Extracellular Vesicles as Mediators in Atherosclerotic Cardiovascular Disease.J Lipid Atheroscler. 2024 Sep;13(3):232-261. doi: 10.12997/jla.2024.13.3.232. Epub 2024 Aug 26. J Lipid Atheroscler. 2024. PMID: 39355407 Free PMC article. Review.
-
Coagulation protease-induced extracellular vesicles: their potential effects on coagulation and inflammation.J Thromb Haemost. 2024 Nov;22(11):2976-2990. doi: 10.1016/j.jtha.2024.07.022. Epub 2024 Aug 8. J Thromb Haemost. 2024. PMID: 39127325 Free PMC article. Review.
-
Dihydrogeodin from Fennellia flavipes Modulates Platelet Aggregation via Downregulation of Calcium Signaling, αIIbβ3 Integrins, MAPK, and PI3K/Akt Pathways.Mar Drugs. 2025 May 17;23(5):212. doi: 10.3390/md23050212. Mar Drugs. 2025. PMID: 40422802 Free PMC article.
-
The protein cargo of extracellular vesicles correlates with the epigenetic aging clock of exercise sensitive DNAmFitAge.Biogerontology. 2025 Jan 8;26(1):35. doi: 10.1007/s10522-024-10177-9. Biogerontology. 2025. PMID: 39775340 Free PMC article.
-
The emerging roles of platelet-derived extracellular vesicles in disease.Ann Med. 2025 Dec;57(1):2499029. doi: 10.1080/07853890.2025.2499029. Epub 2025 May 3. Ann Med. 2025. PMID: 40317251 Free PMC article. Review.
References
-
- Thery C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F, Atkin-Smith GK, Ayre DC, Bach JM, Bachurski D, Baharvand H, Balaj L, Baldacchino S, Bauer NN, Baxter AA, Bebawy M, Beckham C, Bedina Zavec A, Benmoussa A, Berardi AC, Bergese P, Bielska E, Blenkiron C, Bobis-Wozowicz S, Boilard E, Boireau W, Bongiovanni A, Borras FE, Bosch S, Boulanger CM, Breakefield X, Breglio AM, Brennan MA, Brigstock DR, Brisson A, Broekman ML, Bromberg JF, Bryl-Gorecka P, Buch S, Buck AH, Burger D, Busatto S, Buschmann D, Bussolati B, Buzas EI, Byrd JB, Camussi G, Carter DR, Caruso S, Chamley LW, Chang YT, Chen C, Chen S, Cheng L, Chin AR, Clayton A, Clerici SP, Cocks A, Cocucci E, Coffey RJ, Cordeiro-da-Silva A, Couch Y, Coumans FA, Coyle B, Crescitelli R, Criado MF, D'Souza-Schorey C, Das S, Datta Chaudhuri A, de Candia P, De Santana EF, De Wever O, Del Portillo HA, Demaret T, Deville S, Devitt A, Dhondt B, Di Vizio D, Dieterich LC, Dolo V, Dominguez Rubio AP, Dominici M, Dourado MR, Driedonks TA, Duarte FV, Duncan HM, Eichenberger RM, Ekstrom K, El Andaloussi S, Elie-Caille C, Erdbrugger U, Falcon-Perez JM, Fatima F, Fish JE, Flores-Bellver M, Forsonits A, Frelet-Barrand A, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7:1535750. doi: 10.1080/20013078.2018.1535750. - DOI - PMC - PubMed
-
- Badimon L, Suades R, Fuentes E, Palomo I, Padro T. Role of platelet-derived microvesicles as crosstalk mediators in atherothrombosis and future pharmacology targets: a link between inflammation, atherosclerosis, and thrombosis. Front Pharmacol. 2016;7:293. doi: 10.3389/fphar.2016.00293. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- PID2019-107160RB-I00/National Plan for Science and Innovation
- PGC2018-094025-B-I00/National Plan for Science and Innovation
- CB16/11/00411/Centro de Investigación Biomédica en Red Enfermedades Cardiovasculares
- FIS PI19/01687/Instituto de Salud Carlos III
- 2017 SGR 1480/Departament d'Universitats, Recerca i Societat de la Informació
LinkOut - more resources
Full Text Sources

