Nudix hydrolase 15 (NUDT15) loss-of-function variants in an Australian inflammatory bowel disease population
- PMID: 35289057
- PMCID: PMC9796699
- DOI: 10.1111/imj.15746
Nudix hydrolase 15 (NUDT15) loss-of-function variants in an Australian inflammatory bowel disease population
Abstract
Background: Thiopurine-related adverse events such as leukopenia, liver dysfunction and pancreatitis are associated with variants in the NUDT15 gene. Loss-of-function (low or no enzyme activity) alleles are more common in Asian and Hispanic populations. The prevalence of these variants in the Australian inflammatory bowel disease (IBD) population has not yet been reported.
Aim: To evaluate the presence of NUDT15 loss-of-function alleles *2,*3,*9 in the Australian IBD population.
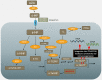
Methods: The NUDT15 screening cohort included 423 IBD patients from Brisbane, Australia. Study patients were recruited by: (i) retrospective review of clinical charts for thiopurine-related severe adverse events; (ii) pathology data (white blood cell (WBC) and neutrophil counts). NUDT15 genotyping was performed using polymerase chain reaction (PCR)-high-resolution melt (HRM), TaqMan genotyping and Sanger sequencing.
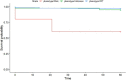
Results: NUDT15 mutation R139C (allele *3) was identified in 8 of 423 (1.9%) IBD patients. Seven of eight patients were R139C heterozygous (C/T) and one patient was R139C homozygous (T/T). One of the C/T group and the T/T patient developed thiopurine-induced myelosuppression (TIM) within 60 days of dosing. One patient in the C/T group developed TIM after 60 days of thiopurine dosing. The remaining five patients in the C/T group did not show TIM; however, other thiopurine-related events could not be ruled out and therefore careful monitoring over a long period is recommended.
Conclusions: This is the first study to report the frequency of NUDT15 haplotypes *2,*3,*9 in an Australian IBD population. The most common variant detected was the R139C mutation. PCR and Sanger sequencing are efficient and cost-effective approaches for NUDT15 genotyping.
Keywords: Crohn disease; NUDT15; inflammatory bowel disease; myelosuppression; thiopurine; ulcerative colitis.
© 2022 The Authors. Internal Medicine Journal published by John Wiley & Sons Australia, Ltd on behalf of Royal Australasian College of Physicians.
Figures
Comment in
-
Author reply.Intern Med J. 2022 Nov;52(11):2018. doi: 10.1111/imj.15953. Intern Med J. 2022. PMID: 36404112 No abstract available.
-
NUDT15 genotype testing in inflammatory bowel disease patients in Australia.Intern Med J. 2022 Nov;52(11):2016-2017. doi: 10.1111/imj.15943. Intern Med J. 2022. PMID: 36404115 No abstract available.
References
-
- Beaugerie L, Brousse N, Bouvier AM, Colombel JF, Lémann M, Cosnes J et al. Lymphoproliferative disorders in patients receiving thiopurines for inflammatory bowel disease: a prospective observational cohort study. Lancet 2009; 374: 1617–25. - PubMed
-
- Ansari A, Hassan C, Duley J, Marinaki A, Shobowale‐Bakre EM, Seed P et al. Thiopurine methyltransferase activity and the use of azathioprine in inflammatory bowel disease. Aliment Pharmacol Ther 2002; 16: 1743–50. - PubMed
-
- Marinaki AM, Ansari A, Duley JA, Arenas M, Sumi S, Lewis CM et al. Adverse drug reactions to azathioprine therapy are associated with polymorphism in the gene encoding inosine triphosphate pyrophosphatase (ITPase). Pharmacogenetics 2004; 14: 181–7. - PubMed
-
- Nielsen OH, Vainer B, Rask‐Madsen J. Review article: the treatment of inflammatory bowel disease with 6‐mercaptopurine or azathioprine. Aliment Pharmacol Ther 2001; 15: 1699–708. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

