Quantitative restoration of immune defense in old animals determined by naive antigen-specific CD8 T-cell numbers
- PMID: 35289071
- PMCID: PMC9009107
- DOI: 10.1111/acel.13582
Quantitative restoration of immune defense in old animals determined by naive antigen-specific CD8 T-cell numbers
Abstract
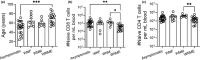
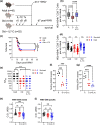
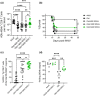
Older humans and animals often exhibit reduced immune responses to infection and vaccination, and this often directly correlates to the numbers and frequency of naive T (Tn) cells. We found such a correlation between reduced numbers of blood CD8+ Tn cells and severe clinical outcomes of West Nile virus (WNV) in both humans naturally exposed to, and mice experimentally infected with, WNV. To examine possible causality, we sought to increase the number of CD8 Tn cells by treating C57BL/6 mice with IL-7 complexes (IL-7C, anti-IL-7 mAb bound to IL-7), shown previously to efficiently increase peripheral T-cell numbers by homeostatic proliferation. T cells underwent robust expansion following IL-7C administration to old mice increasing the number of total T cells (>fourfold) and NS4b:H-2Db -restricted antigen-specific CD8 T cells (twofold). This improved the numbers of NS4b-specific CD8 T cells detected at the peak of the response against WNV, but not survival of WNV challenge. IL-7C-treated old animals also showed no improvement in WNV-specific effector immunity (neutralizing antibody and in vivo T-cell cytotoxicity). To test quantitative limits to which CD8 Tn cell restoration could improve protective immunity, we transferred graded doses of Ag-specific precursors into old mice and showed that injection of 5400 (but not of 1800 or 600) adult naive WNV-specific CD8 T cells significantly increased survival after WNV. These results set quantitative limits to the level of Tn reconstitution necessary to improve immune defense in older organisms and are discussed in light of targets of immune reconstitution.
Keywords: IL-7/ CD8 T cells; immune aging; immune rejuvenation.
© 2022 The Authors. Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
J.N.Ž. is co‐chair of the scientific advisory board of and receives research funding from Young Blood Institute, Inc.
Figures



References
-
- Blattman, J. N. , Antia, R. , Sourdive, D. J. D. , Wang, X. , Kaech, S. M. , Murali‐Krishna, K. , Altman, J. D. , & Ahmed, R. (2002). Estimating the precursor frequency of naive antigen‐specific CD8 T cells. Journal of Experimental Medicine, 195(5), 657–664. 10.1084/jem.20001021 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

