Targeting the vasculature in cardiometabolic disease
- PMID: 35289308
- PMCID: PMC8920329
- DOI: 10.1172/JCI148556
Targeting the vasculature in cardiometabolic disease
Abstract
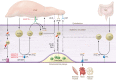
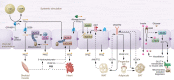
Obesity has reached epidemic proportions and is a major contributor to insulin resistance (IR) and type 2 diabetes (T2D). Importantly, IR and T2D substantially increase the risk of cardiovascular (CV) disease. Although there are successful approaches to maintain glycemic control, there continue to be increased CV morbidity and mortality associated with metabolic disease. Therefore, there is an urgent need to understand the cellular and molecular processes that underlie cardiometabolic changes that occur during obesity so that optimal medical therapies can be designed to attenuate or prevent the sequelae of this disease. The vascular endothelium is in constant contact with the circulating milieu; thus, it is not surprising that obesity-driven elevations in lipids, glucose, and proinflammatory mediators induce endothelial dysfunction, vascular inflammation, and vascular remodeling in all segments of the vasculature. As cardiometabolic disease progresses, so do pathological changes in the entire vascular network, which can feed forward to exacerbate disease progression. Recent cellular and molecular data have implicated the vasculature as an initiating and instigating factor in the development of several cardiometabolic diseases. This Review discusses these findings in the context of atherosclerosis, IR and T2D, and heart failure with preserved ejection fraction. In addition, novel strategies to therapeutically target the vasculature to lessen cardiometabolic disease burden are introduced.
Figures
References
-
- Hales CM, et al. Prevalence of obesity among adults and youth: United States, 2015-2016. NCHS Data Brief. 2017;(288):1–8. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

