Symmetric and asymmetric receptor conformation continuum induced by a new insulin
- PMID: 35289328
- PMCID: PMC9248236
- DOI: 10.1038/s41589-022-00981-0
Symmetric and asymmetric receptor conformation continuum induced by a new insulin
Abstract
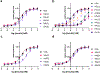
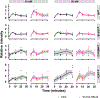
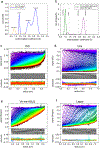
Cone snail venoms contain a wide variety of bioactive peptides, including insulin-like molecules with distinct structural features, binding modes and biochemical properties. Here, we report an active humanized cone snail venom insulin with an elongated A chain and a truncated B chain, and use cryo-electron microscopy (cryo-EM) and protein engineering to elucidate its interactions with the human insulin receptor (IR) ectodomain. We reveal how an extended A chain can compensate for deletion of B-chain residues, which are essential for activity of human insulin but also compromise therapeutic utility by delaying dissolution from the site of subcutaneous injection. This finding suggests approaches to developing improved therapeutic insulins. Curiously, the receptor displays a continuum of conformations from the symmetric state to a highly asymmetric low-abundance structure that displays coordination of a single humanized venom insulin using elements from both of the previously characterized site 1 and site 2 interactions.
© 2022. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
Competing interests
A patent application related to this work was filed by the University of Utah.
Figures

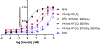
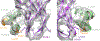

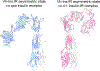
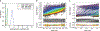

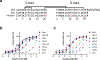

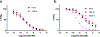
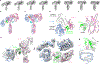
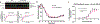
References
-
- Shabanpoor F, Separovic F & Wade JD The human insulin superfamily of polypeptide hormones. Vitam Horm 80, 1–31 (2009). - PubMed
-
- Heinemann L Variability of Insulin Absorption and Insulin Action. Diabetes Technology & Therapeutics 4(2004). - PubMed
-
- Carpenter FH Relationship of structure to biological activity of insulin as revealed by degradative studies. The American Journal of Medicine 40, 750–758 (1966). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

