Electrically Switchable Polymer Brushes for Protein Capture and Release in Biological Environments
- PMID: 35289480
- PMCID: PMC9311814
- DOI: 10.1002/anie.202115745
Electrically Switchable Polymer Brushes for Protein Capture and Release in Biological Environments
Abstract
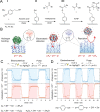
Interfaces functionalized with polymers are known for providing excellent resistance towards biomolecular adsorption and for their ability to bind high amounts of protein while preserving their structure. However, making an interface that switches between these two states has proven challenging and concepts to date rely on changes in the physiochemical environment, which is static in biological systems. Here we present the first interface that can be electrically switched between a high-capacity (>1 μg cm-2 ) multilayer protein binding state and a completely non-fouling state (no detectable adsorption). Switching is possible over multiple cycles without any regeneration. Importantly, switching works even when the interface is in direct contact with biological fluids and a buffered environment. The technology offers many applications such as zero fouling on demand, patterning or separation of proteins as well as controlled release of biologics in a physiological environment, showing high potential for future drug delivery in vivo.
Keywords: Adsorption; Electrochemistry; Polymer Brushes; Proteins.
© 2022 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors G.F.D.dC. and A.D. have filed a patent based on the findings presented here and started the company Nyctea Technologies AB.
Figures


References
-
- None
-
- Cohen Stuart M. A., Huck W. T. S., Genzer J., Muller M., Ober C., Stamm M., Sukhorukov G. B., Szleifer I., Tsukruk V. V., Urban M., Winnik F., Zauscher S., Luzinov I., Minko S., Nat. Mater. 2010, 9, 101–113; - PubMed
-
- Krishnamoorthy M., Hakobyan S., Ramstedt M., Gautrot J. E., Chem. Rev. 2014, 114, 10976–11026; - PubMed
-
- Jain P., Baker G. L., Bruening M. L., Annu. Rev. Anal. Chem. 2009, 2, 387–408; - PubMed
-
- Tokarev I., Minko S., Adv. Funct. Mater. 2020, 30, 1903478;
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

