Tumor marker response to SARS-CoV-2 infection among patients with cancer
- PMID: 35289488
- PMCID: PMC9110907
- DOI: 10.1002/cam4.4646
Tumor marker response to SARS-CoV-2 infection among patients with cancer
Abstract
Background: Inflammatory responses from benign conditions can cause non-cancer-related elevations in tumor markers. The severe acute respiratory coronavirus 2 (SARS-CoV-2) induces a distinct viral inflammatory response, resulting in coronavirus disease 2019 (COVID-19). Clinical data suggest carcinoembryonic antigen (CEA), carbohydrate antigen 19-9 (CA 19-9), and cancer antigen 125 (CA 125) levels might rise in patients with COVID-19. However, available data excludes cancer patients, so little is known about the effect of COVID-19 on tumor markers among cancer patients.
Methods: We conducted a case series and identified patients with a positive SARS-CoV-2 PCR test, diagnosis of a solid tumor malignancy, and a CEA, CA 19-9, CA 125, or CA 27-29 laboratory test. Cancer patients with documented COVID-19 infection and at least one pre- and two post-infection tumor marker measurements were included. We abstracted the electronic health record for demographics, cancer diagnosis, treatment, evidence of cancer progression, date and severity of COVID-19 infection, and tumor marker values.
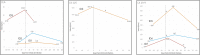
Results: Seven patients were identified with a temporary elevation of tumor marker values during the post-COVID-19 period. Elevation in tumor marker occurred within 56 days of COVID-19 infection for all patients. Tumor markers subsequently decreased at the second time point in the post-infectious period among all patients.
Conclusion: We report temporary elevations of cancer tumor markers in the period surrounding COVID-19 infection. To our knowledge this is the first report of this phenomenon in cancer patients and has implications for clinical management and future research.
Keywords: COVID-19; cancer management; clinical management; clinical observations; tumor markers; viral infection.
© 2022 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
None.
Figures

Similar articles
-
High mortality rate in cancer patients with symptoms of COVID-19 with or without detectable SARS-COV-2 on RT-PCR.Eur J Cancer. 2020 Aug;135:251-259. doi: 10.1016/j.ejca.2020.05.028. Epub 2020 Jun 7. Eur J Cancer. 2020. PMID: 32540204 Free PMC article.
-
Efficacy of hydroxychloroquine for post-exposure prophylaxis to prevent severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection among adults exposed to coronavirus disease (COVID-19): a structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Jun 3;21(1):475. doi: 10.1186/s13063-020-04446-4. Trials. 2020. PMID: 32493478 Free PMC article.
-
Safety and Efficacy of Imatinib for Hospitalized Adults with COVID-19: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Oct 28;21(1):897. doi: 10.1186/s13063-020-04819-9. Trials. 2020. PMID: 33115543 Free PMC article.
-
Pathogenesis-directed therapy of 2019 novel coronavirus disease.J Med Virol. 2021 Mar;93(3):1320-1342. doi: 10.1002/jmv.26610. Epub 2020 Nov 10. J Med Virol. 2021. PMID: 33073355 Review.
-
[SARS-CoV-2 and Microbiological Diagnostic Dynamics in COVID-19 Pandemic].Mikrobiyol Bul. 2020 Jul;54(3):497-509. doi: 10.5578/mb.69839. Mikrobiyol Bul. 2020. PMID: 32755524 Review. Turkish.
Cited by
-
The Potential Role of MUC16 (CA125) Biomarker in Lung Cancer: A Magic Biomarker but with Adversity.Diagnostics (Basel). 2022 Nov 29;12(12):2985. doi: 10.3390/diagnostics12122985. Diagnostics (Basel). 2022. PMID: 36552994 Free PMC article. Review.
-
Surveillance of gynecologic cancer patients post-COVID-19 vaccine: Are CA-125 levels reliable?Gynecol Oncol Rep. 2023 Feb;45:101140. doi: 10.1016/j.gore.2023.101140. Epub 2023 Jan 21. Gynecol Oncol Rep. 2023. PMID: 36714374 Free PMC article.
-
Serum hyaluronic acid and procollagen III, N-terminal propeptide levels are highly associated with disease severity and predict the progression of COVID-19.Front Cell Infect Microbiol. 2023 Oct 4;13:1249038. doi: 10.3389/fcimb.2023.1249038. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37860066 Free PMC article.
References
-
- Network NCC . Colon cancer (version 32021). 2021. Accessed September 15, 2021. https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf
-
- Network NCC . Pancreatic adenocarcinoma (version: 2.2021). 2021. Accessed September 15, 2021. https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf
-
- Network NCC . Breast cancer (version 8.2021). 2021. Accessed September 15, 2021. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf
-
- Network NCC . Ovarian cancer/fallopian tube cancer/primary peritoneal cancer (version: 3.2021). 2021. Accessed September 15, 2021. https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

