Seasonal Fluctuations in Iron Cycling in Thawing Permafrost Peatlands
- PMID: 35290040
- PMCID: PMC9097474
- DOI: 10.1021/acs.est.1c06937
Seasonal Fluctuations in Iron Cycling in Thawing Permafrost Peatlands
Abstract
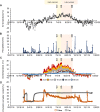
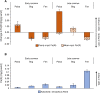
In permafrost peatlands, up to 20% of total organic carbon (OC) is bound to reactive iron (Fe) minerals in the active layer overlying intact permafrost, potentially protecting OC from microbial degradation and transformation into greenhouse gases (GHG) such as CO2 and CH4. During the summer, shifts in runoff and soil moisture influence redox conditions and therefore the balance of Fe oxidation and reduction. Whether reactive iron minerals could act as a stable sink for carbon or whether they are continuously dissolved and reprecipitated during redox shifts remains unknown. We deployed bags of synthetic ferrihydrite (FH)-coated sand in the active layer along a permafrost thaw gradient in Stordalen mire (Abisko, Sweden) over the summer (June to September) to capture changes in redox conditions and quantify the formation and dissolution of reactive Fe(III) (oxyhydr)oxides. We found that the bags accumulated Fe(III) under constant oxic conditions in areas overlying intact permafrost over the full summer season. In contrast, in fully thawed areas, conditions were continuously anoxic, and by late summer, 50.4 ± 12.8% of the original Fe(III) (oxyhydr)oxides were lost via dissolution. Periodic redox shifts (from 0 to +300 mV) were observed over the summer season in the partially thawed areas. This resulted in the dissolution and loss of 47.2 ± 20.3% of initial Fe(III) (oxyhydr)oxides when conditions are wetter and more reduced, and new formation of Fe(III) minerals (33.7 ± 8.6% gain in comparison to initial Fe) in the late summer under more dry and oxic conditions, which also led to the sequestration of Fe-bound organic carbon. Our data suggest that there is seasonal turnover of iron minerals in partially thawed permafrost peatlands, but that a fraction of the Fe pool remains stable even under continuously anoxic conditions.
Keywords: Abisko; Arctic; bioavailability; iron; microbial Fe(III) reduction and Fe(II) oxidation; permafrost collapse; seasonal fluctuations; soil organic carbon.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Schuur E. A. G.; McGuire A. D.; Schadel C.; Grosse G.; Harden J. W.; Hayes D. J.; Hugelius G.; Koven C. D.; Kuhry P.; Lawrence D. M.; Natali S. M.; Olefeldt D.; Romanovsky V. E.; Schaefer K.; Turetsky M. R.; Treat C. C.; Vonk J. E. Climate change and the permafrost carbon feedback. Nature 2015, 520, 171–179. 10.1038/nature14338. - DOI - PubMed
-
- McGuire A. D.; Anderson L. G.; Christensen T. R.; Dallimore S.; Guo L. D.; Hayes D. J.; Heimann M.; Lorenson T. D.; Macdonald R. W.; Roulet N. Sensitivity of the carbon cycle in the Arctic to climate change. Ecol. Monogr. 2009, 79, 523–555. 10.1890/08-2025.1. - DOI
-
- Christensen J. H.; Kanikicharla K. K.; Aldrian E.; An S. I.; Albuquerque Cavalcanti I. F.; de Castro M.; Dong W.; Goswami P.; Hall A.; Kanyanga J. K.; Kitoh A.; Kossin J.; Lau N. C.; Renwick J.; Stephenson D. B.; Xie S. P.; Zhou T.; Abraham L.; Ambrizzi T.; Anderson B.; Arakawa O.; Arritt R.; Baldwin M.; Barlow M.; Barriopedro D.; Biasutti M.; Biner S.; Bromwich D.; Brown J.; Cai W.; Carvalho L. V.; Chang P.; Chen X.; Choi J.; Christensen O. B.; Deser C.; Emanuel K.; Endo H.; Enfield D. B.; Evan A.; Giannini A.; Gillett N.; Hariharasubramanian A.; Huang P.; Jones J.; Karumuri A.; Katzfey J.; Kjellström E.; Knight J.; Knutson T.; Kulkarni A.; Kundeti K. R.; Lau W. K.; Lenderink G.; Lennard C.; Leung L. Y. R.; Lin R.; Losada T.; Mackellar N. C.; Magaña V.; Marshall G.; Mearns L.; Meehl G.; Menéndez C.; Murakami H.; Nath M. J.; Neelin J. D.; van Oldenborgh G. J.; Olesen M.; Polcher J.; Qian Y.; Ray S.; Reich K. D.; de Fonseca B. R.; Ruti P.; Screen J.; Sedláček J.; Solman S.; Stendel M.; Stevenson S.; Takayabu I.; Turner J.; Ummenhofer C.; Walsh K.; Wang B.; Wang C.; Watterson I.; Widlansky M.; Wittenberg A.; Woollings T.; Yeh S. W.; Zhang C.; Zhang L.; Zheng X.; Zou L.. Climate Phenomena and Their Relevance for Future Regional Climate Change. Climate Change 2013 the Physical Science Basis: Working Group I Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press, 2013; pp 1217–1308.
-
- Solomon S.; Qin D.; Manning M.; Chen Z.; Marquis M.; Averyt K. B.; Tignor M.; Miller H. L., Eds. IPCC, Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press, 2007.
-
- Swindles G. T.; Morris P. J.; Mullan D.; Watson E. J.; Turner T. E.; Roland T. P.; Amesbury M. J.; Kokfelt U.; Schoning K.; Pratte S.; Gallego-Sala A.; Charman D. J.; Sanderson N.; Garneau M.; Carrivick J. L.; Woulds C.; Holden J.; Parry L.; Galloway J. M. The long-term fate of permafrost peatlands under rapid climate warming. Sci. Rep. 2016, 5, 17951 10.1038/srep17951. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

