A phase I randomized, double-blind, placebo-controlled study to evaluate the safety, tolerability, and immunogenicity of a live-attenuated quadrivalent dengue vaccine in flavivirus-naïve and flavivirus-experienced healthy adults
- PMID: 35290152
- PMCID: PMC9225326
- DOI: 10.1080/21645515.2022.2046960
A phase I randomized, double-blind, placebo-controlled study to evaluate the safety, tolerability, and immunogenicity of a live-attenuated quadrivalent dengue vaccine in flavivirus-naïve and flavivirus-experienced healthy adults
Abstract
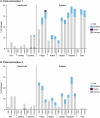
Dengue (DENV) is a mosquito-borne virus with four serotypes causing substantial morbidity in tropical and subtropical areas worldwide. V181 is an investigational, live, attenuated, quadrivalent dengue vaccine. In this phase 1 double-blind, placebo-controlled study, the safety, tolerability, and immunogenicity of V181 in baseline flavivirus-naïve (BFN) and flavivirus-experienced (BFE) healthy adults were evaluated in two formulations: TV003 and TV005. TV005 contains a 10-fold higher DENV2 level than TV003. Two-hundred adults were randomized 2:2:1 to receive TV003, TV005, or placebo on Days 1 and 180. Immunogenicity against the 4 DENV serotypes was measured using a Virus Reduction Neutralization Test (VRNT60) after each vaccination and out to 1 year after the second dose. There were no discontinuations due to adverse events (AE) or serious vaccine-related AEs in the study. Most common AEs after TV003 or TV005 were headache, rash, fatigue, and myalgia. Tri- or tetravalent vaccine-viremia was detected in 63.9% and 25.6% of BFN TV003 and TV005 participants, respectively, post-dose 1 (PD1). Tri- or tetravalent dengue VRNT60 seropositivity was demonstrated in 92.6% of BFN TV003, 74.2% of BFN TV005, and 100% of BFE TV003 and TV005 participants PD1. Increases in VRNT60 GMTs were observed after the first vaccination with TV003 and TV005 in both flavivirus subgroups for all dengue serotypes, and minimal increases were measured PD2. GMTs in the TV003 and TV005 BFE and BFN groups remained above the respective baselines and placebo through 1-year PD2. These data support further development of V181 as a single-dose vaccine for the prevention of dengue disease.
Keywords: Dengue; V181; immunogenicity; phase 1; safety; vaccine.
Conflict of interest statement
K.L.R., A.W.L., T.F., K.C., A.R., M.S., J.M., D.H., S.G-K., A.B. and B.C. are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA and may hold stock in Merck & Co., Inc., Kenilworth, NJ, USA. R.R reports grants or contracts from Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. C.D-P. reports grants or contracts from Takeda Pharmaceutical Company, Leidos, Pfizer Inc., Sanofi and Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. C.A. reports employment at Diagnostic Research Group, which was a site for this research and received payment for performing the study.
Figures






References
-
- Stanaway JD, Shepard DS, Undurraga EA, Halasa YA, Coffeng LE, Brady OJ, Hay SI, Bedi N, Bensenor IM, Castaneda-Orjuela CA, et al. The global burden of dengue: an analysis from the global burden of disease study 2013. Lancet Infect Dis. 2016;16(6):712–23. doi:10.1016/s1473-3099(16)00026-8. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
