Corticofugal regulation of predictive coding
- PMID: 35290181
- PMCID: PMC8983050
- DOI: 10.7554/eLife.73289
Corticofugal regulation of predictive coding
Abstract
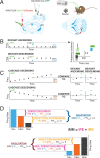
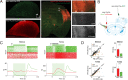
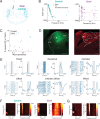
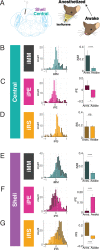
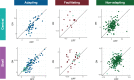
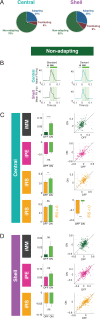
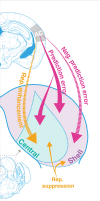
Sensory systems must account for both contextual factors and prior experience to adaptively engage with the dynamic external environment. In the central auditory system, neurons modulate their responses to sounds based on statistical context. These response modulations can be understood through a hierarchical predictive coding lens: responses to repeated stimuli are progressively decreased, in a process known as repetition suppression, whereas unexpected stimuli produce a prediction error signal. Prediction error incrementally increases along the auditory hierarchy from the inferior colliculus (IC) to the auditory cortex (AC), suggesting that these regions may engage in hierarchical predictive coding. A potential substrate for top-down predictive cues is the massive set of descending projections from the AC to subcortical structures, although the role of this system in predictive processing has never been directly assessed. We tested the effect of optogenetic inactivation of the auditory cortico-collicular feedback in awake mice on responses of IC neurons to stimuli designed to test prediction error and repetition suppression. Inactivation of the cortico-collicular pathway led to a decrease in prediction error in IC. Repetition suppression was unaffected by cortico-collicular inactivation, suggesting that this metric may reflect fatigue of bottom-up sensory inputs rather than predictive processing. We also discovered populations of IC units that exhibit repetition enhancement, a sequential increase in firing with stimulus repetition. Cortico-collicular inactivation led to a decrease in repetition enhancement in the central nucleus of IC, suggesting that it is a top-down phenomenon. Negative prediction error, a stronger response to a tone in a predictable rather than unpredictable sequence, was suppressed in shell IC units during cortico-collicular inactivation. These changes in predictive coding metrics arose from bidirectional modulations in the response to the standard and deviant contexts, such that the units in IC responded more similarly to each context in the absence of cortical input. We also investigated how these metrics compare between the anesthetized and awake states by recording from the same units under both conditions. We found that metrics of predictive coding and deviance detection differ depending on the anesthetic state of the animal, with negative prediction error emerging in the central IC and repetition enhancement and prediction error being more prevalent in the absence of anesthesia. Overall, our results demonstrate that the AC provides cues about the statistical context of sound to subcortical brain regions via direct feedback, regulating processing of both prediction and repetition.
Keywords: adaptation; auditory cortex; auditory neuroscience; feedback; inferior colliculus; mouse; neuroscience; predictive coding.
© 2022, Lesicko et al.
Conflict of interest statement
AL, CA, JB, MD, MG No competing interests declared
Figures





References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

