Maternal Exercise-Induced SOD3 Reverses the Deleterious Effects of Maternal High-Fat Diet on Offspring Metabolism Through Stabilization of H3K4me3 and Protection Against WDR82 Carbonylation
- PMID: 35290440
- PMCID: PMC9163554
- DOI: 10.2337/db21-0706
Maternal Exercise-Induced SOD3 Reverses the Deleterious Effects of Maternal High-Fat Diet on Offspring Metabolism Through Stabilization of H3K4me3 and Protection Against WDR82 Carbonylation
Abstract
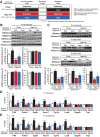
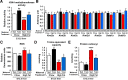
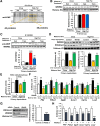
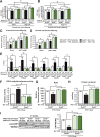
Preclinical studies reveal maternal exercise as a promising intervention to reduce the transmission of multigenerational metabolic dysfunction caused by maternal obesity. The benefits of maternal exercise on offspring health may arise from multiple factors and have recently been shown to involve DNA demethylation of critical hepatic genes leading to enhanced glucose metabolism in offspring. Histone modification is another epigenetic regulator, yet the effects of maternal obesity and exercise on histone methylation in offspring are not known. Here, we find that maternal high-fat diet (HFD; 60% kcal from fat) induced dysregulation of offspring liver glucose metabolism in C57BL/6 mice through a mechanism involving increased reactive oxygen species, WD repeat-containing 82 (WDR82) carbonylation, and inactivation of histone H3 lysine 4 (H3K4) methyltransferase leading to decreased H3K4me3 at the promoters of glucose metabolic genes. Remarkably, the entire signal was restored if the HFD-fed dams had exercised during pregnancy. WDR82 overexpression in hepatoblasts mimicked the effects of maternal exercise on H3K4me3 levels. Placental superoxide dismutase 3 (SOD3), but not antioxidant treatment with N-acetylcysteine was necessary for the regulation of H3K4me3, gene expression, and glucose metabolism. Maternal exercise regulates a multicomponent epigenetic system in the fetal liver resulting in the transmission of the benefits of exercise to offspring.
© 2022 by the American Diabetes Association.
Figures

References
-
- Perng W, Oken E, Dabelea D. Developmental overnutrition and obesity and type 2 diabetes in offspring. Diabetologia 2019;62:1779–1788 - PubMed
-
- Volpato AM, Schultz A, Magalhães-da-Costa E, Correia ML, Águila MB, Mandarim-de-Lacerda CA. Maternal high-fat diet programs for metabolic disturbances in offspring despite leptin sensitivity. Neuroendocrinology 2012;96:272–284 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

