Long-term efficacy and safety of subcutaneous concizumab prophylaxis in hemophilia A and hemophilia A/B with inhibitors
- PMID: 35290453
- PMCID: PMC9198939
- DOI: 10.1182/bloodadvances.2021006403
Long-term efficacy and safety of subcutaneous concizumab prophylaxis in hemophilia A and hemophilia A/B with inhibitors
Abstract
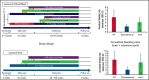
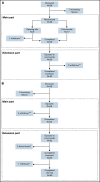
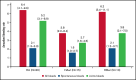
Despite current therapies, there remains an unmet need for treatment for patients with hemophilia. The main parts of two phase 2 trials established clinical proof-of-concept for once-daily, subcutaneous concizumab prophylaxis in patients with hemophilia A/B with inhibitors (HAwI/HBwI; explorer4) and severe hemophilia A without inhibitors (HA; explorer5). Here, we present results from extension parts of these trials, included to evaluate longer term safety and efficacy. Both trials included main (≥24 weeks) and extension (52-102 weeks) parts, with patients receiving concizumab 0.15 mg/kg with potential dose escalation to concizumab 0.20 or 0.25 mg/kg if they experienced ≥3 treated spontaneous bleeding episodes within 12 weeks. Endpoints included annualized bleeding rate (ABR), adverse events (AEs), and occurrence of antidrug antibodies. Thromboembolic events were AEs of special interest. Thirty-six patients with HA, 15 with HAwI, and 10 with HBwI were exposed to concizumab. Estimated ABRs during the main + extension parts at last dose level were 4.8 (95% confidence interval [CI], 3.2-7.2) and 6.4 (95% CI, 4.1-9.9) in explorer4 and explorer5, respectively (spontaneous ABRs were 1.8 [95% CI, 1.2-2.6] and 2.1 [95% CI, 1.3-3.3]). Most AEs were mild, with no deaths, events leading to withdrawal, or thromboembolic events. Anti-drug antibodies developed in 25% of patients and were low titer and transient, with no observed clinical effect in most cases. Results of the main + extension parts of these trials were consistent with results of the main parts. Ongoing phase 3 trials will further evaluate concizumab as a once-daily, subcutaneous treatment across hemophilia subtypes. These trials were registered at www.clinicaltrials.gov as #NCT03196284 and #NCT03196297.
© 2022 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Figures

References
-
- Srivastava A, Santagostino E, Dougall A, et al. . WFH guidelines for the management of hemophilia, 3rd edition. Haemophilia. 2020;26(suppl 6): 1-158. - PubMed
-
- Berntorp E, Dolan G, Hay C, et al. . European retrospective study of real-life haemophilia treatment. Haemophilia. 2017;23(1):105-114. - PubMed
-
- van den Berg HM, Fischer K, Carcao M, et al. ; PedNet Study Group . Timing of inhibitor development in more than 1000 previously untreated patients with severe hemophilia A. Blood. 2019;134(3):317-320. - PubMed

