Systemic quinolones and risk of retinal detachment III: a nested case-control study using a US electronic health records database
- PMID: 35290480
- PMCID: PMC9107393
- DOI: 10.1007/s00228-021-03260-4
Systemic quinolones and risk of retinal detachment III: a nested case-control study using a US electronic health records database
Abstract
Background: Quinolones are popular antibiotics that are known for their potency, broad coverage, and reasonable safety. Concerns have been raised about a possible association between quinolones and retinal detachment (RD).
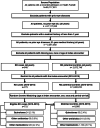
Methods: We conducted a nested case-control study using electronic health records (EHR) from the Health Facts® Database. The initial cohort included all patients who were admitted between 2000 and 2016, with no history of eye disease, and had a minimum medical history of one year. Eligible cases comprised inpatients who were first admitted with a primary diagnosis of RD between 2010 and 2015. Each eligible case was matched without replacement to five unique controls by sex, race, age, and period-at-risk. We used conditional logistic regression to calculate RD risk, adjusting for exposure to other medications, and major risk factors.
Results: We identified 772 cases and 3860 controls. Whereas our primary analysis of all subjects revealed no quinolone-associated RD risk, elevated but non-significant risks were noted in African Americans (ciprofloxacin and levofloxacin), those aged 56-70 years old (moxifloxacin), and women (ciprofloxacin).
Conclusion: Our study did not identify an elevated RD risk within 30 days following systemic administration of quinolone antibiotics. Suggestions of increased risk observed in some population subgroups warrant further investigation.
Keywords: Drug safety; Electronic health records; Nested case–control study; Pharmacovigilance; Quinolones; Retinal detachment.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Systemic quinolones and risk of acute liver failure III: A nested case-control study using a US electronic health records database.J Gastroenterol Hepatol. 2021 Aug;36(8):2307-2314. doi: 10.1111/jgh.15504. Epub 2021 Mar 31. J Gastroenterol Hepatol. 2021. PMID: 33755266 Free PMC article.
-
Systemic quinolones and risk of retinal detachment I: analysis of data from the US FDA adverse event reporting system.Expert Opin Drug Saf. 2022 Feb;21(2):269-276. doi: 10.1080/14740338.2022.1993187. Epub 2021 Oct 24. Expert Opin Drug Saf. 2022. PMID: 34641748
-
Exposure to oral fluoroquinolones and the risk of retinal detachment: retrospective analyses of two large healthcare databases.Drug Saf. 2014 Mar;37(3):171-82. doi: 10.1007/s40264-014-0138-y. Drug Saf. 2014. PMID: 24526267 Free PMC article.
-
Safety issues and drug-drug interactions with commonly used quinolones.Expert Opin Drug Metab Toxicol. 2015 Jan;11(1):25-39. doi: 10.1517/17425255.2014.970166. Epub 2014 Nov 26. Expert Opin Drug Metab Toxicol. 2015. PMID: 25423877 Review.
-
Tamponade in surgery for retinal detachment associated with proliferative vitreoretinopathy.Cochrane Database Syst Rev. 2020 May 13;5(5):CD006126. doi: 10.1002/14651858.CD006126.pub4. Cochrane Database Syst Rev. 2020. PMID: 32408387 Free PMC article.
Cited by
-
Safety of fluoroquinolones.Rev Esp Quimioter. 2024 Apr;37(2):127-133. doi: 10.37201/req/143.2023. Epub 2023 Dec 22. Rev Esp Quimioter. 2024. PMID: 38140798 Free PMC article. Review.
-
Association between fluoroquinolones and retinal detachment: insights from a large German health claims-based cohort study.BMC Ophthalmol. 2025 Aug 7;25(1):447. doi: 10.1186/s12886-025-04284-5. BMC Ophthalmol. 2025. PMID: 40775308 Free PMC article.
-
Use of fluoroquinolones and risk of rhegmatogenous retinal detachment: a retrospective cohort study using two nationwide representative claims databases.Front Pharmacol. 2024 Dec 11;15:1414221. doi: 10.3389/fphar.2024.1414221. eCollection 2024. Front Pharmacol. 2024. PMID: 39723254 Free PMC article.
-
Overview of Side-Effects of Antibacterial Fluoroquinolones: New Drugs versus Old Drugs, a Step Forward in the Safety Profile?Pharmaceutics. 2023 Mar 1;15(3):804. doi: 10.3390/pharmaceutics15030804. Pharmaceutics. 2023. PMID: 36986665 Free PMC article. Review.
References
-
- Transparency Market Research (2014) Antibacterial drugs market expected to reach USD 45.09 billion globally in 2019: Transparency Market Research. In: ed
-
- Emmerson A, Jones A (2003) The quinolones: decades of development and use The Journal of antimicrobial chemotherapy 51 (Suppl. S1): 13–20 - PubMed
-
- Furiex (2012) Novel Fluoroquinolone (JNJ-Q2). In: ed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous