Randomized Clinical Trial of High-Dose Rifampicin With or Without Levofloxacin Versus Standard of Care for Pediatric Tuberculous Meningitis: The TBM-KIDS Trial
- PMID: 35291004
- PMCID: PMC9617573
- DOI: 10.1093/cid/ciac208
Randomized Clinical Trial of High-Dose Rifampicin With or Without Levofloxacin Versus Standard of Care for Pediatric Tuberculous Meningitis: The TBM-KIDS Trial
Abstract
Background: Pediatric tuberculous meningitis (TBM) commonly causes death or disability. In adults, high-dose rifampicin may reduce mortality. The role of fluoroquinolones remains unclear. There have been no antimicrobial treatment trials for pediatric TBM.
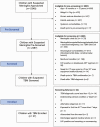
Methods: TBM-KIDS was a phase 2 open-label randomized trial among children with TBM in India and Malawi. Participants received isoniazid and pyrazinamide plus: (i) high-dose rifampicin (30 mg/kg) and ethambutol (R30HZE, arm 1); (ii) high-dose rifampicin and levofloxacin (R30HZL, arm 2); or (iii) standard-dose rifampicin and ethambutol (R15HZE, arm 3) for 8 weeks, followed by 10 months of standard treatment. Functional and neurocognitive outcomes were measured longitudinally using Modified Rankin Scale (MRS) and Mullen Scales of Early Learning (MSEL).
Results: Of 2487 children prescreened, 79 were screened and 37 enrolled. Median age was 72 months; 49%, 43%, and 8% had stage I, II, and III disease, respectively. Grade 3 or higher adverse events occurred in 58%, 55%, and 36% of children in arms 1, 2, and 3, with 1 death (arm 1) and 6 early treatment discontinuations (4 in arm 1, 1 each in arms 2 and 3). By week 8, all children recovered to MRS score of 0 or 1. Average MSEL scores were significantly better in arm 1 than arm 3 in fine motor, receptive language, and expressive language domains (P < .01).
Conclusions: In a pediatric TBM trial, functional outcomes were excellent overall. The trend toward higher frequency of adverse events but better neurocognitive outcomes in children receiving high-dose rifampicin requires confirmation in a larger trial.
Clinical trials registration: NCT02958709.
Keywords: clinical trial; high-dose rifampicin; levofloxacin; neuropsychological; pediatric tuberculous meningitis.
© The Author(s) 2022. Published by Oxford University Press for the Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. A. G. reports funding received by her institution outside the scope of this work from NIH, Unitaid, the Centers for Disease Control and Prevention (CDC), and various foundations (Wyncote, Ujala, Todi); participation on a data safety monitoring board or advisory board for the NIH/NIAID Advisory Council and Indo US Science Technology Governing Board; and a leadership or fiduciary role on the IMPAACT Network TB Scientific Committee and the World Health Organization (WHO) multidrug-resistant tuberculosis guidelines committee. D. B. D., K. Y. K., M. V., P. G., and S. B. report support outside the scope of this work given to the ICMR-NIRT for the TBM-KIDS trial by the NICHD and funded through John Hopkins University. K. T. T. reports grants or contracts (NIH, CDC), outside the scope of this work, and consulting fees from WHO. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures