Human Induced Pluripotent Stem Cells: From Cell Origin, Genomic Stability, and Epigenetic Memory to Translational Medicine
- PMID: 35291013
- PMCID: PMC9216482
- DOI: 10.1093/stmcls/sxac020
Human Induced Pluripotent Stem Cells: From Cell Origin, Genomic Stability, and Epigenetic Memory to Translational Medicine
Abstract
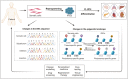
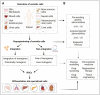
The potential of human induced pluripotent stem cells (iPSCs) to self-renew indefinitely and to differentiate virtually into any cell type in unlimited quantities makes them attractive for in vitro disease modeling, drug screening, personalized medicine, and regenerative therapies. As the genome of iPSCs thoroughly reproduces that of the somatic cells from which they are derived, they may possess genetic abnormalities, which would seriously compromise their utility and safety. Genetic aberrations could be present in donor somatic cells and then transferred during iPSC generation, or they could occur as de novo mutations during reprogramming or prolonged cell culture. Therefore, to warrant the safety of human iPSCs for clinical applications, analysis of genetic integrity, particularly during iPSC generation and differentiation, should be carried out on a regular basis. On the other hand, reprogramming of somatic cells to iPSCs requires profound modifications in the epigenetic landscape. Changes in chromatin structure by DNA methylations and histone tail modifications aim to reset the gene expression pattern of somatic cells to facilitate and establish self-renewal and pluripotency. However, residual epigenetic memory influences the iPSC phenotype, which may affect their application in disease therapeutics. The present review discusses the somatic cell origin, genetic stability, and epigenetic memory of iPSCs and their impact on basic and translational research.
Keywords: epigenetic memory; genetic aberrations; genetic stability; human induced pluripotent stem cells; point mutations; somatic origin.
© The Author(s) 2022. Published by Oxford University Press.
Figures


Similar articles
-
Epigenetic memory in induced pluripotent stem cells.Nature. 2010 Sep 16;467(7313):285-90. doi: 10.1038/nature09342. Nature. 2010. PMID: 20644535 Free PMC article.
-
Epigenetic regulation of somatic cell reprogramming.Curr Opin Genet Dev. 2017 Oct;46:156-163. doi: 10.1016/j.gde.2017.07.002. Epub 2017 Aug 17. Curr Opin Genet Dev. 2017. PMID: 28823984 Review.
-
Redox and Epigenetics in Human Pluripotent Stem Cells Differentiation.Antioxid Redox Signal. 2021 Feb 1;34(4):335-349. doi: 10.1089/ars.2019.7983. Epub 2020 Jul 17. Antioxid Redox Signal. 2021. PMID: 32567336
-
The genomic stability of induced pluripotent stem cells.Protein Cell. 2012 Apr;3(4):271-7. doi: 10.1007/s13238-012-2922-8. Epub 2012 Apr 19. Protein Cell. 2012. PMID: 22528751 Free PMC article. Review.
-
The Epigenetic Reprogramming Roadmap in Generation of iPSCs from Somatic Cells.J Genet Genomics. 2015 Dec 20;42(12):661-70. doi: 10.1016/j.jgg.2015.10.001. Epub 2015 Oct 23. J Genet Genomics. 2015. PMID: 26743984 Review.
Cited by
-
The dynamics of chromatin states mediated by epigenetic modifications during somatic cell reprogramming.Front Cell Dev Biol. 2023 Jan 16;11:1097780. doi: 10.3389/fcell.2023.1097780. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 36727112 Free PMC article. Review.
-
Cell Reprogramming, Transdifferentiation, and Dedifferentiation Approaches for Heart Repair.Int J Mol Sci. 2025 Mar 27;26(7):3063. doi: 10.3390/ijms26073063. Int J Mol Sci. 2025. PMID: 40243729 Free PMC article. Review.
-
CTCF deletion alters the pluripotency and DNA methylation profile of human iPSCs.Front Cell Dev Biol. 2023 Nov 30;11:1302448. doi: 10.3389/fcell.2023.1302448. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 38099298 Free PMC article.
-
Revitalizing the Epigenome of Adult Jaw Periosteal Cells: Enhancing Diversity in iPSC-Derived Mesenchymal Stem Cells (iMSCs).Cells. 2025 Apr 22;14(9):627. doi: 10.3390/cells14090627. Cells. 2025. PMID: 40358151 Free PMC article.
-
Vascular organoids: unveiling advantages, applications, challenges, and disease modelling strategies.Stem Cell Res Ther. 2023 Oct 10;14(1):292. doi: 10.1186/s13287-023-03521-2. Stem Cell Res Ther. 2023. PMID: 37817281 Free PMC article. Review.
References
-
- Yu JY, Vodyanik MA, Smuga-Otto K, et al. . Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):1917-1920. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

