Curcumin-incorporated 3D bioprinting gelatin methacryloyl hydrogel reduces reactive oxygen species-induced adipose-derived stem cell apoptosis and improves implanting survival in diabetic wounds
- PMID: 35291229
- PMCID: PMC8918758
- DOI: 10.1093/burnst/tkac001
Curcumin-incorporated 3D bioprinting gelatin methacryloyl hydrogel reduces reactive oxygen species-induced adipose-derived stem cell apoptosis and improves implanting survival in diabetic wounds
Abstract
Background: Gelatin methacryloyl (GelMA) hydrogels loaded with stem cells have proved to be an effective clinical treatment for wound healing. Advanced glycation end product (AGE), interacting with its particular receptor (AGER), gives rise to reactive oxygen species (ROS) and apoptosis. Curcumin (Cur) has excellent antioxidant activity and regulates intracellular ROS production and apoptosis. In this study, we developed a Cur-incorporated 3D-printed GelMA to insert into adipose-derived stem cells (ADSCs) and applied it to diabetic wounds.
Methods: GelMA hydrogels with Cur were fabricated and their in vitro effects on ADSCs were investigated. We used structural characterization, western blot, ROS and apoptosis assay to evaluate the antioxidant and anti-apoptotic activity, and assessed the wound healing effects to investigate the mechanism underlying regulation of apoptosis by Cur via the AGE/AGER/nuclear factor-κB (NF-κB) p65 pathway.
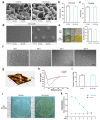
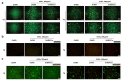
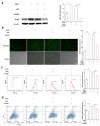
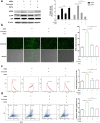
Results: A 10% GelMA scaffold exhibited appropriate mechanical properties and biocompatibility for ADSCs. The circular mesh structure demonstrated printability of 10% GelMA and Cur-GelMA bioinks. The incorporation of Cur into the 10% GelMA hydrogel showed an inhibitory effect on AGEs/AGER/NF-κB p65-induced ROS generation and ADSC apoptosis. Furthermore, Cur-GelMA scaffold promoted cell survival and expedited in vivo diabetic wound healing.
Conclusions: The incorporation of Cur improved the antioxidant activity of 3D-printed GelMA hydrogel and mitigated AGE/AGER/p65 axis-induced ROS and apoptosis in ADSCs. The effects of scaffolds on wound healing suggested that Cur/GelMA-ADSC hydrogel could be an effective biological material for accelerating wound healing.
Keywords: 3D printing; Adipose-derived stem cells; Advanced glycation end products; Curcumin; GelMA; Wound healing.
© The Author(s) 2022. Published by Oxford University Press.
Figures

References
-
- Rathmann W, Giani G. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:2568–9author reply 9. - PubMed
-
- Game F. Treatment strategies for neuroischaemic diabetic foot ulcers. Lancet Diabetes Endocrinol. 2018;6:159–60. - PubMed
-
- Eke G, Mangir N, Hasirci N, MacNeil S, Hasirci V. Development of a UV crosslinked biodegradable hydrogel containing adipose derived stem cells to promote vascularization for skin wounds and tissue engineering. Biomaterials. 2017;129:188–98. - PubMed
LinkOut - more resources
Full Text Sources

