Fragmentation and Ionization Efficiency of Positional and Functional Isomers of Paeoniflorin Derivatives in Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry
- PMID: 35291502
- PMCID: PMC8900252
- DOI: 10.5702/massspectrometry.A0101
Fragmentation and Ionization Efficiency of Positional and Functional Isomers of Paeoniflorin Derivatives in Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry
Abstract
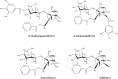
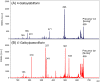
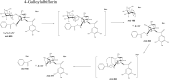
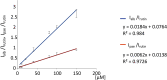
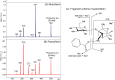
Paeoniflorin and albiflorin, which are functional isomers, are the major constituents of an herbal medicine derived from Paeonia lactiflora. Those functional isomers and their galloylated derivatives, which are positional isomers, were studied by matrix-assisted laser desorption/ionization-tandem mass spectrometry (MALDI-MS/MS). The resulting mass spectra are discussed based on the fragmentation patterns of the sodium adducts. The product ion spectra of 4-O-galloylalbiflorin and 4'-O-galloylpaeoniflorin differed, even though they were positional isomers. The fragmentations of the ester parts were influenced by the neighboring hydroxyl groups. The ionization efficiency of the sodium adduct of albiflorin was higher than that for paeoniflorin. These results indicate that the carboxylic ester group has a higher affinity for sodium ions than the acetal group, which can be attributed to the carbonyl oxygen being negatively polarized, allowing it to function as a Lewis base.
Keywords: MS/MS; albiflorin; fragmentation; ionization efficiency; paeoniflorin.
Copyright © 2022 Tohru Yamagaki, Kohtaro Sugahara, Kohki Fujikawa, and Kazuto Washida.
Figures

References
-
- K. Terasawa. in Economic and Medical Plant Research (Eds.: H. Wagner, N. R. Farnsworth), Academic Press, San Diego, 1999, p. 57.
-
- M. Kaneda, Y. Iitaka, S. Shibata. Chemical studies on the oriental plant drugs—XXXIII: The absolute structures of paeoniflorin, albiflorin, oxypaeoniflorin and benzoylpaeoniflorin isolated from Chinese paeony root. Tetrahedron 28: 4309–4317, 1972.
-
- N. Murakami, M. Saka, H. Shimada, H. Matsuda, J. Yamahara, M. Yoshikawa. New bioactive monoterpene glycosides from Paeoniae Radix. Chem. Pharm. Bull. 44: 1279–1281, 1996. - PubMed
-
- K. Washida, T. Yamagaki, T. Iwashita, K. Nomoto. Two new galloylated monoterpene glycosides, 4-O-galloyl albiflorin and 4′-O-galloylpaeoniflorin, from the roots of Paeonia lactiflora (Paeoniae Radix) grown and processed in Nara Prefecture, Japan. Chem. Pharm. Bull. 57: 1150–1152, 2009. - PubMed
-
- K. Washida, Y. Itoh, T. Iwashita, K. Nomoto. Androgen modulators from the roots of Paeonia lactiflora (Paeoniae Radix) grown and processed in Nara Prefecture, Japan. Chem. Pharm. Bull. 57: 971–974, 2009. - PubMed

