Immunotherapy Effectiveness in Treating Peanut Hypersensitivity: A Systemic Review
- PMID: 35291522
- PMCID: PMC8896406
- DOI: 10.7759/cureus.21832
Immunotherapy Effectiveness in Treating Peanut Hypersensitivity: A Systemic Review
Abstract
Peanut hypersensitivity is one of the top causes of food-related allergic responses and death in high-income countries. As a result, the goal of this study was to see if various forms of immunotherapies can help reduce the severity of peanut hypersensitivity reactions. From 2019 to 2021, a systematic search of PubMed, Web of Science, Wiley online library, and Science Direct was done. Peanut immunotherapy (PIT) clinical trials were considered. There were 19 trials with a total of 1565 participants. Twelve were on oral immunotherapy (OIT), two on sublingual immunotherapy (SLIT), two on subcutaneous immunotherapy (SCIT), two on epicutaneous immunotherapy (EPIT), and one was a comparison of SLIT and OIT. Desensitization was achieved by 74.3% of those who received OIT, 11% of those who received SLIT, 61% of those who received SCIT, and 49% of those who received EPIT. The majority of adverse events (AE) were mild to moderate. Those requiring epinephrine, on the other hand, were moderate to severe and were more common in the therapy groups. This systematic review showed that the current PIT regimens can accomplish desensitization regardless of the route of administration, with an acceptable safety profile.
Keywords: anaphylaxis; desensitization; immunotherapy; non-randomized clinical trial; peanut hypersensitivity; randomized clinical trial.
Copyright © 2022, Alghamdi et al.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
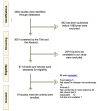
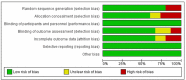
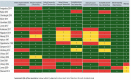
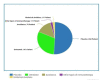
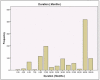
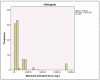
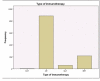
References
-
- Further fatalities caused by anaphylactic reactions to food, 2001-2006. Bock SA, Muñoz-Furlong A, Sampson HA. https://pubmed.ncbi.nlm.nih.gov/17306354/ J Allergy Clin Immunol. 2007;119:1016–1018. - PubMed
-
- Food allergy: a practice parameter update-2014. Sampson HA, Aceves S, Bock SA, et al. https://pubmed.ncbi.nlm.nih.gov/25174862/ J Allergy Clin Immunol. 2014;134:1016–1025. - PubMed
-
- EAACI food allergy and anaphylaxis guidelines: diagnosis and management of food allergy. Muraro A, Werfel T, Hoffmann-Sommergruber K, et al. https://onlinelibrary.wiley.com/doi/10.1111/all.12429. Allergy. 2014;69:1008–1025. - PubMed
-
- The natural history of peanut allergy. Skolnick HS, Conover-Walker MK, Koerner CB, Sampson HA, Burks W, Wood RA. https://www.jacionline.org/article/S0091-6749(01)48941-X/fulltext. J Allergy Clin Immunol. 2001;107:367–374. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
