Concanavalin A-induced autoimmune hepatitis model in mice: Mechanisms and future outlook
- PMID: 35291566
- PMCID: PMC8886606
- DOI: 10.1515/biol-2022-0013
Concanavalin A-induced autoimmune hepatitis model in mice: Mechanisms and future outlook
Abstract
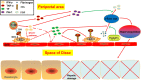
The concanavalin A (Con A)-induced liver injury mouse model is a typical animal model focusing on T cell-dependent hepatic damage in the field of autoimmune hepatitis (AIH). However, the underlying mechanism of hepatic dysfunction due to cell activation or signaling pathways triggered by Con A has not been fully clarified. Therefore, the controversy on this model remains in the academic community. In this article, we first summarized the merit and demerit of this contentious model from the perspectives of cell dysfunction, microcirculation disturbance, involved signaling pathways, as well as the properties of Con A. Then, we summed up the scientific implications of the model in elucidating the pathogenesis of AIH, and the shortcomings of this model were also summarized to elucidate the pathogenesis and application prospect of this classical liver injury mouse model in the study of AIH.
Keywords: autoimmune hepatitis; concanavalin A; experimental animal models; inflammation; pathogenesis.
© 2022 Yang Liu et al., published by De Gruyter.
Conflict of interest statement
Conflict of interest: Authors state no conflict of interest.
Figures


Similar articles
-
Pathogenesis of Concanavalin A induced autoimmune hepatitis in mice.Int Immunopharmacol. 2022 Jan;102:108411. doi: 10.1016/j.intimp.2021.108411. Epub 2021 Dec 8. Int Immunopharmacol. 2022. PMID: 34891001 Review.
-
Immune mechanisms of Concanavalin A model of autoimmune hepatitis.World J Gastroenterol. 2012 Jan 14;18(2):119-25. doi: 10.3748/wjg.v18.i2.119. World J Gastroenterol. 2012. PMID: 22253517 Free PMC article. Review.
-
Concanavalin-A as a Model for Induction of Murine Autoimmune Hepatitis: Role of TNF-α and NF-κβ During The Acute Phase.Egypt J Immunol. 2020 Jun;27(2):19-30. Egypt J Immunol. 2020. PMID: 33548974
-
Regulatory T Cells in Autoimmune Hepatitis: Unveiling Their Roles in Mouse Models and Patients.Front Immunol. 2020 Oct 7;11:575572. doi: 10.3389/fimmu.2020.575572. eCollection 2020. Front Immunol. 2020. PMID: 33117375 Free PMC article. Review.
-
The farnesyltransferase inhibitor tipifarnib protects against autoimmune hepatitis induced by Concanavalin A.Int Immunopharmacol. 2020 Jun;83:106462. doi: 10.1016/j.intimp.2020.106462. Epub 2020 Apr 3. Int Immunopharmacol. 2020. PMID: 32251961
Cited by
-
Current Treatment Regimens and Promising Molecular Therapies for Chronic Hepatobiliary Diseases.Biomolecules. 2025 Jan 14;15(1):121. doi: 10.3390/biom15010121. Biomolecules. 2025. PMID: 39858515 Free PMC article. Review.
-
Negative Influence of Aging on Differentiation and Proliferation of CD8+ T-Cells in Dogs.Vet Sci. 2023 Aug 25;10(9):541. doi: 10.3390/vetsci10090541. Vet Sci. 2023. PMID: 37756063 Free PMC article.
-
Garcinone E Mitigates Oxidative Inflammatory Response and Protects against Experimental Autoimmune Hepatitis via Modulation of Nrf2/HO-1, NF-κB and TNF-α/JNK Axis.Nutrients. 2022 Dec 21;15(1):16. doi: 10.3390/nu15010016. Nutrients. 2022. PMID: 36615674 Free PMC article.
-
Manganese Exacerbates ConA-Induced Liver Inflammation via the cGAS-STING Signaling Pathway.Inflammation. 2024 Feb;47(1):333-345. doi: 10.1007/s10753-023-01912-4. Epub 2023 Oct 8. Inflammation. 2024. PMID: 37805951
-
Guizhi Fuling Wan ameliorates concanavalin A-induced autoimmune hepatitis in mice.Biomed J. 2025 Feb;48(1):100731. doi: 10.1016/j.bj.2024.100731. Epub 2024 Apr 26. Biomed J. 2025. PMID: 38677491 Free PMC article.
References
-
- Valgeirsson KB, Hreinsson JP, Björnsson ES. Increased incidence of autoimmune hepatitis is associated with wider use of biological drugs. Liver Int: Off J Int Assoc Study Liver. 2019;39(12):2341–9. - PubMed
- Valgeirsson KB, Hreinsson JP, Björnsson ES. Increased incidence of autoimmune hepatitis is associated with wider use of biological drugs. . Liver Int: Off J Int Assoc Study Liver. 2019;39(12):2341–9. - PubMed
-
- Sharma R, Verna EC, Söderling J, Roelstraete B, Hagström H, Jonas F, et al. Increased mortality risk in autoimmune hepatitis: a nationwide population-based cohort study with histopathology. Clin Gastroenterol Hepatol. 2020;S1542–3565(20):31395. - PMC - PubMed
- Sharma R, Verna EC, Söderling J, Roelstraete B, Hagström H, Jonas F. et al. Increased mortality risk in autoimmune hepatitis: a nationwide population-based cohort study with histopathology. Clin Gastroenterol Hepatol. 2020;S1542–3565(20):31395. - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
