Clinical Significance of Circulating Serum Levels of sCD95 and TNF-α in Cytoprotection of Cervical Cancer
- PMID: 35291617
- PMCID: PMC8903371
- DOI: 10.52547/rbmb.10.4.711
Clinical Significance of Circulating Serum Levels of sCD95 and TNF-α in Cytoprotection of Cervical Cancer
Abstract
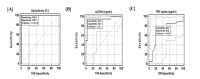
Background: This study correlates the serum levels of sCD95 & TNF-α with a simple cell-based assay to evaluate the capacity of the serum sample to induce apoptosis in Jurkat cells. Interlinking of these parameters can be explored to design a minimum invasive diagnostic strategy for cervical cancer (CC).
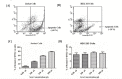
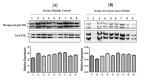
Methods: Sera samples were assessed to induce apoptosis in Jurkat cells through FACS. Serum levels of sCD95 and TNF-α were measured by ELISA. JNK phosphorylation was evaluated in sera incubated Jurkat cells. Data was scrutinized through statistical analysis.
Results: Significantly higher serum levels of sCD95 and lower TNF-α levels were observed in CC patients; their sera samples inhibited induction of apoptosis in Jurkat cells through reduced JNK phosphorylation. Statistical analysis linked these three parameters for the early screening of CC.
Conclusion: Distinct sera levels of sCD95 & TNF-α in CC patients showed an anti-apoptotic effect, which can be considered for early detection of CC.
Keywords: Apoptosis; Jurkat Cells; Tumor Necrosis Factor-alpha; Uterine Cervical Neoplasms; sCD95.
Figures


Similar articles
-
Apoptosis induction in Jurkat cells and sCD95 levels in women's sera are related with the risk of developing cervical cancer.BMC Cancer. 2008 Apr 11;8:99. doi: 10.1186/1471-2407-8-99. BMC Cancer. 2008. PMID: 18405371 Free PMC article.
-
Pulmonary lesions and serum levels of soluble Fas (sCD95) in former hard coal miners.Eur J Med Res. 2010 Nov 4;15 Suppl 2(Suppl 2):60-3. doi: 10.1186/2047-783x-15-s2-60. Eur J Med Res. 2010. PMID: 21147622 Free PMC article.
-
Serum levels of soluble CD95 are not associated with amelioration of multiple sclerosis during pregnancy.J Neurol Sci. 2007 Jan 15;252(1):83-7. doi: 10.1016/j.jns.2006.10.013. Epub 2006 Dec 12. J Neurol Sci. 2007. PMID: 17169375
-
Soluble forms of CD95 and CD95 ligand after living related liver transplantation.Transplantation. 1999 Feb 27;67(4):634-6. doi: 10.1097/00007890-199902270-00026. Transplantation. 1999. PMID: 10071041
-
Soluble FAS (CD95) is not elevated in the serum of patients with myeloid leukemias, myeloproliferative and myelodysplastic syndromes.Leukemia. 1996 Sep;10(9):1531-3. Leukemia. 1996. PMID: 8751476
Cited by
-
Expression Level of lncRNA CYTOR in Iranian Cervical Cancer Patients.Rep Biochem Mol Biol. 2023 Apr;12(1):120-126. doi: 10.52547/rbmb.12.1.120. Rep Biochem Mol Biol. 2023. PMID: 37724154 Free PMC article.
References
-
- Danial NN, Korsmeyer SJ. Cell Death: Critical Control Points. Cell. 2004;116(2):205–19. - PubMed
-
- Walczak H, Krammer PH. The CD95 (APO-1/Fas) and the TRAIL (APO-2L) apoptosis systems. Exp Cell Res. 2000 Apr;256(1):58–66. - PubMed
-
- Townson JL, Naumov GN, Chambers AF. The role of apoptosis in tumor progression and metastasis. Curr Mol Med. 2003 Nov;3(7):631–42. - PubMed
-
- Igney FH, Krammer PH. Immune escape of tumors: apoptosis resistance and tumor counterattack. J Leukoc Biol. 2002;71(6):907–20. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
