Early diagnosis of breast cancer lung metastasis by nanoprobe-based luminescence imaging of the pre-metastatic niche
- PMID: 35292019
- PMCID: PMC8922882
- DOI: 10.1186/s12951-022-01346-4
Early diagnosis of breast cancer lung metastasis by nanoprobe-based luminescence imaging of the pre-metastatic niche
Abstract
Background: Early detection of breast cancer lung metastasis remains highly challenging, due to few metastatic cancer cells at an early stage. Herein we propose a new strategy for early diagnosis of lung metastasis of breast cancer by luminescence imaging of pulmonary neutrophil infiltration via self-illuminating nanoprobes.
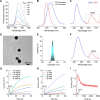
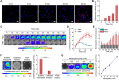
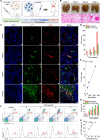
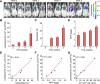
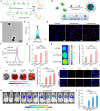
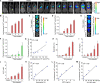
Methods: Luminescent nanoparticles (LAD NPs) were engineered using a biocompatible, neutrophil-responsive self-illuminating cyclodextrin material and an aggregation-induced emission agent. The chemiluminescence resonance energy transfer (CRET) effect and luminescence properties of LAD NPs were fully characterized. Using mouse peritoneal neutrophils, in vitro luminescence properties of LAD NPs were thoroughly examined. In vivo luminescence imaging and correlation analyses were performed in mice inoculated with 4T1 cancer cells. Moreover, an active targeting nanoprobe was developed by surface decoration of LAD NPs with a neutrophil-targeting peptide, which was also systemically evaluated by in vitro and in vivo studies.
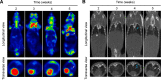
Results: LAD NPs can generate long-wavelength and persistent luminescence due to the CRET effect. In a mouse model of 4T1 breast cancer lung metastasis, we found desirable correlation between neutrophils and tumor cells in the lungs, demonstrating the effectiveness of early imaging of the pre-metastatic niche by the newly developed LAD NPs. The active targeting nanoprobe showed further enhanced luminescence imaging capability for early detection of pulmonary metastasis. Notably, the targeting nanoprobe-based luminescence imaging strategy remarkably outperformed PET/CT imaging modalities in the examined mouse model. Also, preliminary tests demonstrated good safety of LAD NPs.
Conclusions: The neutrophil-targeting imaging strategy based on newly developed luminescence nanoparticles can serve as a promising modality for early diagnosis of lung metastasis of breast cancers.
Keywords: Breast cancer; Luminescence imaging; Lung metastasis; Neutrophils; Targeting nanoprobe.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Duggan C, Trapani D, Ilbawi AM, Fidarova E, Laversanne M, Curigliano G, et al. National health system characteristics, breast cancer stage at diagnosis, and breast cancer mortality: a population-based analysis. Lancet Oncol. 2021;22:1632–42. - PubMed
-
- DeSantis CE, Ma J, Gaudet MM, Newman LA, Miller KD, Goding Sauer A, et al. Breast cancer statistics, 2019. Cancer J Clin. 2019;69:438–51. - PubMed
-
- Harbeck N, Penault-Llorca F, Cortes J, Gnant M, Houssami N, Poortmans P, et al. Breast cancer. Nat Rev Dis Primers. 2019;5:66. - PubMed
-
- Peinado H, Zhang H, Matei IR, Costa-Silva B, Hoshino A, Rodrigues G, et al. Pre-metastatic niches: organ-specific homes for metastases. Nat Rev Cancer. 2017;17:302–17. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

