CYP1B1-catalyzed 4-OHE2 promotes the castration resistance of prostate cancer stem cells by estrogen receptor α-mediated IL6 activation
- PMID: 35292057
- PMCID: PMC8922936
- DOI: 10.1186/s12964-021-00807-x
CYP1B1-catalyzed 4-OHE2 promotes the castration resistance of prostate cancer stem cells by estrogen receptor α-mediated IL6 activation
Abstract
Background: Resistance to androgen deprivation therapy remains a major challenge for the clinical treatment of patients with castration-resistant prostate cancer (CRPC). CYP1B1, a critical enzyme that catalyzes the conversion of estradiol to 4-Hydroxy-17β-estradiol (4-OHE2), has been reported to promote the development and progression of hormone-related cancer, but its role in CRPC is unclear.
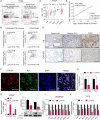
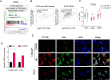
Methods: To explore the underlying mechanism which CYP1B1 promotes the prostate cancer stem cells (PCSCs) characteristics, bioinformatics analyses of human clinical prostate cancer (PCa) datasets were performed. CYP1B1, IL6, and estrogen receptor-α (ERα) expression levels were evaluated in PCa and CRPC tissues via immunohistochemistry. The high-performance liquid chromatography-mass spectrometry assay was carried out to examine intracellular 4-OHE2 levels. Serum-free suspension culture and flow cytometry assays were performed to evaluate PCSCs. Chromatin immunoprecipitation was used to validate that 4-OHE2 recruited ERα to the IL6 promoter.
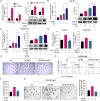
Results: CYP1B1 expression was significantly increased in CRPC tissues and androgen-independent PCa cell lines. CYP1B1+ PCa cells were significantly enriched in bicalutamide-treated LNCaP cells, and CYP1B1 knockdown reduced the cell viability under bicalutamide treatment. In addition, CYP1B1 knockdown decreased the intracellular 4-OHE2 concentration, accompanied by reduced PCSC characteristics. In PCa cells, 4-OHE2 stimulated ERα transcriptional activity and upregulated the expression of IL6 and downstream genes of the IL6-STAT3 signaling. 4-OHE2 increased cell viability under bicalutamide treatment and promoted PCSC characteristics, while IL6 neutralizing antibody reversed these effects. Mechanistically, siERα and the ER antagonist ICI182780 significantly attenuated 4-OHE2-induced IL6 expression, and 4-OHE2 promoted the binding of ERα to the estrogen response element of the IL6 promoter.
Conclusions: Our findings indicate that CYP1B1-catalyzed 4-OHE2 enhanced PCSC characteristics and attenuated bicalutamide sensitivity by ERα-mediated the IL6-STAT3 pathway activation. Our study further emphasizes the role of CYP1B1 in castration resistance and illustrates a novel mechanism of CRPC development. Video Abstract.
Keywords: 4-OHE2; CYP1B1; Castration-resistant prostate cancer; ERα; IL6-STAT3 pathway; PCSC.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
The ERα-NRF2 signalling axis promotes bicalutamide resistance in prostate cancer.Cell Commun Signal. 2022 Nov 14;20(1):178. doi: 10.1186/s12964-022-00979-0. Cell Commun Signal. 2022. PMID: 36376959 Free PMC article.
-
Aromatase-induced endogenous estrogen promotes tumour metastasis through estrogen receptor-α/matrix metalloproteinase 12 axis activation in castration-resistant prostate cancer.Cancer Lett. 2019 Dec 28;467:72-84. doi: 10.1016/j.canlet.2019.09.001. Epub 2019 Sep 6. Cancer Lett. 2019. PMID: 31499120
-
The HeyL-Aromatase Axis Promotes Cancer Stem Cell Properties by Endogenous Estrogen-Induced Autophagy in Castration-Resistant Prostate Cancer.Front Oncol. 2022 Jan 12;11:787953. doi: 10.3389/fonc.2021.787953. eCollection 2021. Front Oncol. 2022. PMID: 35096586 Free PMC article.
-
Hypoxia induced cancer stem cell enrichment promotes resistance to androgen deprivation therapy in prostate cancer.Steroids. 2019 Dec;152:108497. doi: 10.1016/j.steroids.2019.108497. Epub 2019 Sep 12. Steroids. 2019. PMID: 31521707 Review.
-
Androgen receptors in hormone-dependent and castration-resistant prostate cancer.Pharmacol Ther. 2013 Dec;140(3):223-38. doi: 10.1016/j.pharmthera.2013.07.003. Epub 2013 Jul 13. Pharmacol Ther. 2013. PMID: 23859952 Review.
Cited by
-
SFRP1 induces a stem cell phenotype in prostate cancer cells.Front Cell Dev Biol. 2023 Mar 9;11:1096923. doi: 10.3389/fcell.2023.1096923. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 36968194 Free PMC article.
-
Genetic Signatures for Distinguishing Chemo-Sensitive from Chemo-Resistant Responders in Prostate Cancer Patients.Curr Issues Mol Biol. 2024 Mar 11;46(3):2263-2277. doi: 10.3390/cimb46030145. Curr Issues Mol Biol. 2024. PMID: 38534761 Free PMC article.
-
STAT3 and Its Pathways' Dysregulation-Underestimated Role in Urological Tumors.Cells. 2022 Sep 27;11(19):3024. doi: 10.3390/cells11193024. Cells. 2022. PMID: 36230984 Free PMC article. Review.
-
CYP1B1 promotes colorectal cancer liver metastasis by enhancing the growth of metastatic cancer cells via a fatty acids-dependent manner.J Gastrointest Oncol. 2023 Dec 31;14(6):2448-2465. doi: 10.21037/jgo-23-895. Epub 2023 Dec 27. J Gastrointest Oncol. 2023. PMID: 38196537 Free PMC article.
-
Cytochrome P450 genes expression in human prostate cancer.Mol Genet Metab Rep. 2024 Jan 9;38:101049. doi: 10.1016/j.ymgmr.2024.101049. eCollection 2024 Mar. Mol Genet Metab Rep. 2024. PMID: 38469085 Free PMC article.
References
-
- Zhou Y, Bolton EC, Jones JO. Androgens and androgen receptor signaling in prostate tumorigenesis. J Mol Endocrinol. 2015;54:R15–29. - PubMed
-
- Konoshenko MY, Bryzgunova OE, Laktionov PP. miRNAs and androgen deprivation therapy for prostate cancer. Biochim Biophys Acta Rev Cancer. 2021;1876:188625. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

