Aggregating human judgment probabilistic predictions of the safety, efficacy, and timing of a COVID-19 vaccine
- PMID: 35292162
- PMCID: PMC8882426
- DOI: 10.1016/j.vaccine.2022.02.054
Aggregating human judgment probabilistic predictions of the safety, efficacy, and timing of a COVID-19 vaccine
Abstract
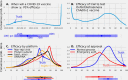
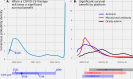
Safe, efficacious vaccines were developed to reduce the transmission of SARS-CoV-2 during the COVID-19 pandemic. But in the middle of 2020, vaccine effectiveness, safety, and the timeline for when a vaccine would be approved and distributed to the public was uncertain. To support public health decision making, we solicited trained forecasters and experts in vaccinology and infectious disease to provide monthly probabilistic predictions from July to September of 2020 of the efficacy, safety, timing, and delivery of a COVID-19 vaccine. We found, that despite sparse historical data, a linear pool-a combination of human judgment probabilistic predictions-can quantify the uncertainty in clinical significance and timing of a potential vaccine. The linear pool underestimated how fast a therapy would show a survival benefit and the high efficacy of approved COVID-19 vaccines. However, the linear pool did make an accurate prediction for when a vaccine would be approved by the FDA. Compared to individual forecasters, the linear pool was consistently above the median of the most accurate forecasts. A linear pool is a fast and versatile method to build probabilistic predictions of a developing vaccine that is robust to poor individual predictions. Though experts and trained forecasters did underestimate the speed of development and the high efficacy of a SARS-CoV-2 vaccine, linear pool predictions can improve situational awareness for public health officials and for the public make clearer the risks, rewards, and timing of a vaccine.
Keywords: COVID-19; Forecasting; Human judgement; Vaccine.
Copyright © 2022 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

