Orally-active, clinically-translatable senolytics restore α-Klotho in mice and humans
- PMID: 35292270
- PMCID: PMC9034457
- DOI: 10.1016/j.ebiom.2022.103912
Orally-active, clinically-translatable senolytics restore α-Klotho in mice and humans
Abstract
Background: α-Klotho is a geroprotective protein that can attenuate or alleviate deleterious changes with ageing and disease. Declines in α-Klotho play a role in the pathophysiology of multiple diseases and age-related phenotypes. Pre-clinical evidence suggests that boosting α-Klotho holds therapeutic potential. However, readily clinically-translatable, practical strategies for increasing α-Klotho are not at hand. Here, we report that orally-active, clinically-translatable senolytics can increase α-Klotho in mice and humans.
Methods: We examined α-Klotho expression in three different human primary cell types co-cultured with conditioned medium (CM) from senescent or non-senescent cells with or without neutralizing antibodies. We assessed α-Klotho expression in aged, obese, and senescent cell-transplanted mice treated with vehicle or senolytics. We assayed urinary α-Klotho in patients with idiopathic pulmonary fibrosis (IPF) who were treated with the senolytic drug combination, Dasatinib plus Quercetin (D+Q).
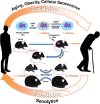
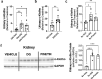
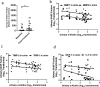
Findings: We found exposure to the senescent cell secretome reduces α-Klotho in multiple nonsenescent human cell types. This was partially prevented by neutralizing antibodies against the senescence-associated secretory phenotype (SASP) factors, activin A and Interleukin 1α (IL-1α). Consistent with senescent cells' being a cause of decreased α-Klotho, transplanting senescent cells into younger mice reduced brain and urine α-Klotho. Selectively removing senescent cells genetically or pharmacologically increased α-Klotho in urine, kidney, and brain of mice with increased senescent cell burden, including naturally-aged, diet-induced obese (DIO), or senescent cell-transplanted mice. D+Q increased α-Klotho in urine of patients with IPF, a disease linked to cellular senescence.
Interpretation: Senescent cells cause reduced α-Klotho, partially due to their production of activin A and IL-1α. Targeting senescent cells boosts α-Klotho in mice and humans. Thus, clearing senescent cells restores α-Klotho, potentially opening a novel, translationally-feasible avenue for developing orally-active small molecule, α-Klotho-enhancing clinical interventions. Furthermore, urinary α-Klotho may prove to be a useful test for following treatments in senolytic clinical trials.
Funding: This work was supported by National Institute of Health grants AG013925 (J.L.K.), AG062413 (J.L.K., S.K.), AG044271 (N.M.), AG013319 (N.M.), and the Translational Geroscience Network (AG061456: J.L.K., T.T., N.M., S.B.K., S.K.), Robert and Arlene Kogod (J.L.K.), the Connor Group (J.L.K.), Robert J. and Theresa W. Ryan (J.L.K.), and the Noaber Foundation (J.L.K.). The previous IPF clinical trial was supported by the Claude D. Pepper Older Americans Independence Centers at WFSM (AG021332: J.N.J., S.B.K.), UTHSCA (AG044271: A.M.N.), and the Translational Geroscience Network.
Keywords: Cellular senescence; Senolytics; α-Klotho.
Copyright © 2022 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests Y.Z., T.T., N.G., T.P., A.K.P., and J.L.K. have a financial interest related to this research. Patents on senolytic drugs are held by Mayo Clinic. This research has been reviewed by the Mayo Clinic Conflict of Interest Review Board and was conducted in compliance with Mayo Clinic Conflict of Interest policies. No conflicts of interest, financial or otherwise, are declared by the other authors.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

