Introduction of Triple-Drug Therapy for Accelerating Lymphatic Filariasis Elimination in India: Lessons Learned
- PMID: 35292580
- PMCID: PMC9154644
- DOI: 10.4269/ajtmh.21-0964
Introduction of Triple-Drug Therapy for Accelerating Lymphatic Filariasis Elimination in India: Lessons Learned
Abstract
There are 670 million people at risk of contracting lymphatic filariasis (LF) in India, which bears 40% of the global burden of the disease. The National Program to Eliminate LF was launched in 2004 first with a single-drug therapy-diethylcarbamazine (DEC), followed by a two-drug therapy-DEC + albendazole (DA). In 2017, following successful drug trials, World Health Organization endorsed a new triple-drug therapy to fight LF using ivermectin with DEC and albendazole (IDA). 1 In June 2018, India made new commitments to accelerate their program to eliminate LF and initiated the new IDA protocol in five districts in the country. This article looks at the experience of India in the roll out of the new drug protocol and shares their preparations, successes, challenges, and lessons learned.
Figures

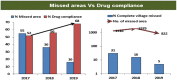

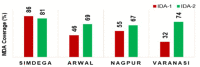




References
-
- World Health Organization , 2017. Guideline: Alternative Mass Drug Administrations to Eliminate Lymphatic Filariasis. Geneva, Switzerland: WHO. Available at: http://apps.who.int/iris/bitstream/handle/10665/259381/9789241550161-eng.... Accessed August 16, 2021. - PubMed
-
- National Vector Borne Disease Control Programme, Directorate General of Health Services, Ministry of Health and Family Welfare, Government of India , 2018. Accelerated Plan for the Elimination of Lymphatic Filariasis. Available at: https://nvbdcp.gov.in/WriteReadData/l892s/1031567531528881007.pdf, page 8. Accessed August 8, 2021.
-
- World Health Organization , 2020. Global programme to eliminate lymphatic filariasis: progress report, 2019. Wkly Epidemiol Rec 43: 513. Available at: https://apps.who.int/iris/bitstream/handle/10665/336185/WER9543-eng-fre.pdf. Accessed July 8, 2021.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

