Exploration and functionalization of M1-macrophage extracellular vesicles for effective accumulation in glioblastoma and strong synergistic therapeutic effects
- PMID: 35292619
- PMCID: PMC8924195
- DOI: 10.1038/s41392-022-00894-3
Exploration and functionalization of M1-macrophage extracellular vesicles for effective accumulation in glioblastoma and strong synergistic therapeutic effects
Abstract
Glioblastoma multiforme (GBM) is a highly aggressive brain tumor with an extremely low survival rate. New and effective approaches for treatment are therefore urgently needed. Here, we successfully developed M1-like macrophage-derived extracellular vesicles (M1EVs) that overcome multiple challenges via guidance from two macrophage-related observations in clinical specimens from GBM patients: enrichment of M2 macrophages in GBM; and origination of a majority of infiltrating macrophage from peripheral blood. To maximize the synergistic effect, we further functionalized the membranes of M1EVs with two hydrophobic agents (the chemical excitation source CPPO (C) and the photosensitizer Ce6 (C)) and loaded the hydrophilic hypoxia-activated prodrug AQ4N (A) into the inner core of the M1EVs. After intravenous injection, the inherent nature of M1-derived extracellular vesicles CCA-M1EVs allowed for blood-brain barrier penetration, and modulated the immunosuppressive tumor microenvironment via M2-to-M1 polarization, which increased hydrogen peroxide (H2O2) levels. Furthermore, the reaction between H2O2 and CPPO produced chemical energy, which could be used for Ce6 activation to generate large amounts of reactive oxygen species to achieve chemiexcited photodynamic therapy (CDT). As this reaction consumed oxygen, the aggravation of tumor hypoxia also led to the conversion of non-toxic AQ4N into toxic AQ4 for chemotherapy. Therefore, CCA-M1EVs achieved synergistic immunomodulation, CDT, and hypoxia-activated chemotherapy in GBM to exert a potent therapeutic effect. Finally, we demonstrated the excellent effect of CCA-M1EVs against GBM in cell-derived xenograft and patient-derived xenograft models, underscoring the strong potential of our highly flexible M1EVs system to support multi-modal therapies for difficult-to-treat GBM.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


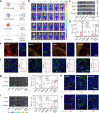

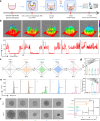


References
-
- Zou Y, et al. Single siRNA Nanocapsules for Effective siRNA Brain Delivery and Glioblastoma Treatment. Adv. Mater. 2020;32:e2000416. - PubMed
-
- Zheng M, et al. ROS-Responsive Polymeric siRNA Nanomedicine Stabilized by Triple Interactions for the Robust Glioblastoma Combinational RNAi Therapy. Adv. Mater. 2019;31:e1903277. - PubMed
-
- Qiao C, et al. Rabies Virus-Inspired Metal-Organic Frameworks (MOFs) for Targeted Imaging and Chemotherapy of Glioma. Angew. Chem. 2020;59:16982–16988. - PubMed
-
- Polson, E. S. et al. KHS101 disrupts energy metabolism in human glioblastoma cells and reduces tumor growth in mice. Sci. Transl. Med. 10, eaar2718 (2018). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

