The possible role of hypoxia in the affected tissue of relapsed clubfoot
- PMID: 35292718
- PMCID: PMC8924187
- DOI: 10.1038/s41598-022-08519-z
The possible role of hypoxia in the affected tissue of relapsed clubfoot
Abstract
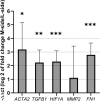
Our aim was to study the expression of hypoxia-related proteins as a possible regulatory pathway in the contracted side tissue of relapsed clubfoot. We compared the expression of hypoxia-related proteins in the tissue of the contracted (medial) side of relapsed clubfoot, and in the tissue of the non-contracted (lateral) side of relapsed clubfoot. Tissue samples from ten patients were analyzed by immunohistochemistry and image analysis, Real-time PCR and Mass Spectrometry to evaluate the differences in protein composition and gene expression. We found a significant increase in the levels of smooth muscle actin, transforming growth factor-beta, hypoxia-inducible factor 1 alpha, lysyl oxidase, lysyl oxidase-like 2, tenascin C, matrix metalloproteinase-2, matrix metalloproteinase-9, fibronectin, collagen types III and VI, hemoglobin subunit alpha and hemoglobin subunit beta, and an overexpression of ACTA2, FN1, TGFB1, HIF1A and MMP2 genes in the contracted medial side tissue of clubfoot. In the affected tissue, we have identified an increase in the level of hypoxia-related proteins, together with an overexpression of corresponding genes. Our results suggest that the hypoxia-associated pathway is potentially a factor contributing to the etiology of clubfoot relapses, as it stimulates both angioproliferation and fibroproliferation, which are considered to be key factors in the progression and development of relapses.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

