Pseudouridine-modified tRNA fragments repress aberrant protein synthesis and predict leukaemic progression in myelodysplastic syndrome
- PMID: 35292784
- PMCID: PMC8924001
- DOI: 10.1038/s41556-022-00852-9
Pseudouridine-modified tRNA fragments repress aberrant protein synthesis and predict leukaemic progression in myelodysplastic syndrome
Abstract
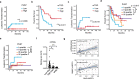
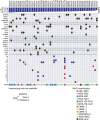
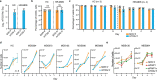
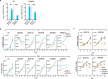
Transfer RNA-derived fragments (tRFs) are emerging small noncoding RNAs that, although commonly altered in cancer, have poorly defined roles in tumorigenesis1. Here we show that pseudouridylation (Ψ) of a stem cell-enriched tRF subtype2, mini tRFs containing a 5' terminal oligoguanine (mTOG), selectively inhibits aberrant protein synthesis programmes, thereby promoting engraftment and differentiation of haematopoietic stem and progenitor cells (HSPCs) in patients with myelodysplastic syndrome (MDS). Building on evidence that mTOG-Ψ targets polyadenylate-binding protein cytoplasmic 1 (PABPC1), we employed isotope exchange proteomics to reveal critical interactions between mTOG and functional RNA-recognition motif (RRM) domains of PABPC1. Mechanistically, this hinders the recruitment of translational co-activator PABPC1-interacting protein 1 (PAIP1)3 and strongly represses the translation of transcripts sharing pyrimidine-enriched sequences (PES) at the 5' untranslated region (UTR), including 5' terminal oligopyrimidine tracts (TOP) that encode protein machinery components and are frequently altered in cancer4. Significantly, mTOG dysregulation leads to aberrantly increased translation of 5' PES messenger RNA (mRNA) in malignant MDS-HSPCs and is clinically associated with leukaemic transformation and reduced patient survival. These findings define a critical role for tRFs and Ψ in difficult-to-treat subsets of MDS characterized by high risk of progression to acute myeloid leukaemia (AML).
© 2022. The Author(s).
Conflict of interest statement
C.B. is founder and member of the scientific advisory board of SACRA Therapeutics. S.M. is a founder and Chief Executive Officer of SACRA Therapeutics. All the other authors declare no competing interests.
Figures









References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

