Nanotechnology-Driven Cell-Based Therapies in Regenerative Medicine
- PMID: 35292878
- PMCID: PMC9074705
- DOI: 10.1208/s12248-022-00692-3
Nanotechnology-Driven Cell-Based Therapies in Regenerative Medicine
Abstract
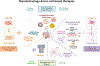
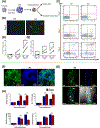
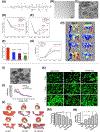
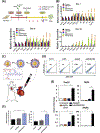
The administration of cells as therapeutic agents has emerged as a novel approach to complement the use of small molecule drugs and other biologics for the treatment of numerous conditions. Although the use of cells for structural and/or functional tissue repair and regeneration provides new avenues to address increasingly complex disease processes, it also faces numerous challenges related to efficacy, safety, and translational potential. Recent advances in nanotechnology-driven cell therapies have the potential to overcome many of these issues through precise modulation of cellular behavior. Here, we describe several approaches that illustrate the use of different nanotechnologies for the optimization of cell therapies and discuss some of the obstacles that need to be overcome to allow for the widespread implementation of nanotechnology-based cell therapies in regenerative medicine.
Keywords: direct cell reprogramming; nanotechnology; nanotransfection; regenerative medicine; stem cell therapy.
© 2022. The Author(s), under exclusive licence to American Association of Pharmaceutical Scientists.
Conflict of interest statement
Conflict of Interest Statement:
The authors have no conflicts of interest to declare.
Figures



References
-
- Wang M-L. The Modern Pharmaceutical Industry: History, Current Position and Challenges. Global Health Partnerships: The Pharmaceutical Industry and BRICA. London: Palgrave Macmillan UK; 2009. p. 33–80.
-
- Mason C, Brindley DA, Culme-Seymour EJ, Davie NL. Cell therapy industry: billion dollar global business with unlimited potential. Regen Med. 2011;6(3):265–72. - PubMed
-
- Au P, Hursh DA, Lim A, Moos MC Jr., Oh SS, Schneider BS, et al. FDA oversight of cell therapy clinical trials. Sci Transl Med. 2012;4(149):149fs31. - PubMed
-
- Towards advanced cell therapies. Nature Biomedical Engineering. 2018;2(6):339–40. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

