A Reference Standard for Analytical Testing of Erythropoietin
- PMID: 35292912
- PMCID: PMC8986685
- DOI: 10.1007/s11095-022-03213-1
A Reference Standard for Analytical Testing of Erythropoietin
Abstract
Purpose: Erythropoietin (EPO) is a 165 amino acid protein that promotes the proliferation of erythrocytic progenitors. A decrease in endogenous EPO production causes anemia that can be treated with recombinant Human EPO (rHuEPO).
Objective: To ensure the safety and efficacy of the rHuEPO, manufacturers must use analytical methods to demonstrate similarity across batches and between different products. To do this they need reference standards to validate their equipment and methods.
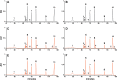
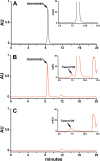
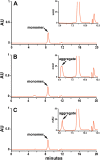
Method: We used peptide mapping, size-exclusion chromatography, glycoprofiling, and isoelectric focusing to analyze a rHuEPO reference standard.
Results: Characterization demonstrates that our rHuEPO reference standard meets the criteria for quality.
Conclusion: The rHuEPO reference standard is fit for purpose as a tool for validating system suitability and methods.
Keywords: Biologics; erythropoietin; lyophilization; manufacturing; reference standards.
© 2022. The Author(s).
Figures


Similar articles
-
Recombinant human erythropoietin in the anemia associated with multiple myeloma or non-Hodgkin's lymphoma: dose finding and identification of predictors of response.Blood. 1995 Dec 15;86(12):4446-53. Blood. 1995. PMID: 8541533 Clinical Trial.
-
Recombinant human erythropoietin in the treatment of multiple myeloma-associated anemia.Acta Haematol. 1997;98(4):204-10. doi: 10.1159/000203625. Acta Haematol. 1997. PMID: 9401498 Clinical Trial.
-
Recombinant human erythropoietin in the treatment of cancer and chemotherapy-induced anemia: results of double-blind and open-label follow-up studies.Semin Oncol. 1994 Apr;21(2 Suppl 3):21-8. Semin Oncol. 1994. PMID: 8202722 Clinical Trial.
-
Erythropoietin hyporesponsiveness: from iron deficiency to iron overload.Kidney Int Suppl. 1999 Mar;69:S107-18. Kidney Int Suppl. 1999. PMID: 10084294 Review.
-
[Recombinant erythropoietin--a fundamental change in the treatment of anemia?].Cesk Pediatr. 1992 Apr;47(4):220-5. Cesk Pediatr. 1992. PMID: 1628358 Review. Slovak.
Cited by
-
Cellulose functionalized magnetic beads for high throughput glycosylation analysis in biotherapeutic modalities.Sci Rep. 2024 Nov 29;14(1):29735. doi: 10.1038/s41598-024-80649-y. Sci Rep. 2024. PMID: 39613820 Free PMC article.
References
-
- WHO (2016) Guidelines on evaluation of monoclonal antibodies as similar biotherapeutic products (SBPs). In: Standardization ECoB, editor. Expert Committee on Biological Standardization. Geneva: World Health Organization; 1–30.
-
- EMA (2018) Guideline on non-clinical and clinical development of similar biolgic medicinal products containing recombinant erythropoietins. In: Use CfMPfH, editor. Product-specific biosimilar guidelines. London, UK: European Medicines Agency; 1–8.
-
- FDA: Biologics Products & Establishments (2021) https://www.fda.gov/vaccines-blood-biologics/biologics-products-establis.... Accessed 4 Nov 2021.
-
- FDA . Development of therapeutic protein Biosimilars: comparative analytical assessment and other quality-related considerations. Guidance for industry. Washington DC: Center for Biologics Evaluation and Research (CBER); 2019. pp. 1–31.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

