Regulation of antral follicular growth by an interplay between gonadotropins and their receptors
- PMID: 35292926
- PMCID: PMC9050977
- DOI: 10.1007/s10815-022-02456-6
Regulation of antral follicular growth by an interplay between gonadotropins and their receptors
Abstract
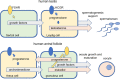
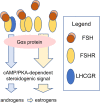
Knowledge of the growth and maturation of human antral follicles is based mainly on concepts and deductions from clinical observations and animal models. To date, new experimental approaches and in vitro data contributed to a deep comprehension of gonadotropin receptors' functioning and may provide new insights into the mechanisms regulating still unclear physiological events. Among these, the production of androgen in the absence of proper LH levels, the programming of follicular atresia and dominance are some of the most intriguing. Starting from evolutionary issues at the basis of the gonadotropin receptor signal specificity, we draw a new hypothesis explaining the molecular mechanisms of the antral follicular growth, based on the modulation of endocrine signals by receptor-receptor interactions. The "heteromer hypothesis" explains how opposite death and life signals are delivered by gonadotropin receptors and other membrane partners, mediating steroidogenesis, apoptotic events, and the maturation of the dominant follicle.
Keywords: Antral follicle; Atresia; FSH; GPER; Heteromers; LH.
© 2022. The Author(s), under exclusive licence to Springer Science+Business Media, LLC, part of Springer Nature.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Simoni M, Gromoll J, Nieschlag E. The follicle-stimulating hormone receptor: biochemistry, molecular biology, physiology, and pathophysiology. Endocr Rev. 1997;18:739–773. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

