Efficient Production of Adipic Acid by a Two-Step Catalytic Reaction of Biomass-Derived 2,5-Furandicarboxylic Acid
- PMID: 35293137
- PMCID: PMC9323459
- DOI: 10.1002/cssc.202200375
Efficient Production of Adipic Acid by a Two-Step Catalytic Reaction of Biomass-Derived 2,5-Furandicarboxylic Acid
Abstract
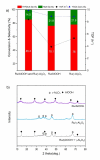
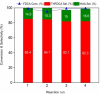
Efficient catalytic ring-opening coupled with hydrogenation is a promising but challenging reaction for producing adipic acid (AA) from 2,5-furan dicarboxylic acid (FDCA). In this study, AA synthesis is carried out in two steps from FDCA via tetrahydrofuran-2,5-dicarboxylic acid (THFDCA) over a recyclable Ru/Al2 O3 and an ionic liquid, [MIM(CH2 )4 SO3 H]I (MIM=methylimidazolium) to deliver 99 % overall yield of AA. Ru/Al2 O3 is found to be an efficient catalyst for hydrogenation and hydrogenolysis of FDCA to deliver THFDCA and 2-hydroxyadipic acid (HAA), respectively, where ruthenium is more economically viable than well-known palladium or rhodium hydrogenation catalysts. H2 chemisorption shows that the alumina phase strongly affects the interaction between Ru nanoparticles (NPs) and supports, resulting in materials with high dispersion and small size of Ru NPs, which in turn are responsible for the high conversion of FDCA. An ionic liquid system, [MIM(CH2 )4 SO3 H]I is applied to the hydrogenolysis of THFDCA for AA production. The [MIM(CH2 )4 SO3 H]I exhibits superior activity, enables simple product isolation with high purity, and reduces the severe corrosion problems caused by the conventional hydroiodic acid catalytic system.
Keywords: biomass valorization; carboxylic acids, heterogeneous catalysis; hydrodeoxygenation; ruthenium.
© 2022 The Authors. ChemSusChem published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









References
-
- None
-
- Wei L., Zhang J., Deng W., Xie S., Zhang Q., Wang Y., Chem. Commun. 2019, 55, 8013–8016; - PubMed
-
- Vardon D. R., Franden M. A., Johnson C. W., Karp E. M., Guarnieri M. T., Linger J. G., Salm M. J., Strathmann T. J., Beckham G. T., Energy Environ. Sci. 2015, 8, 617–628;
-
- Van de Vyver S., Román-Leshkov Y., Catal. Sci. Technol. 2013, 3, 1465–1479;
-
- Beerthuis R., Rothenberg G., Shiju N. R., Green Chem. 2015, 17, 1341–1361;
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

