Normalization of optical fluence distribution for three-dimensional functional optoacoustic tomography of the breast
- PMID: 35293163
- PMCID: PMC8923705
- DOI: 10.1117/1.JBO.27.3.036001
Normalization of optical fluence distribution for three-dimensional functional optoacoustic tomography of the breast
Abstract
Significance: In three-dimensional (3D) functional optoacoustic tomography (OAT), wavelength-dependent optical attenuation and nonuniform incident optical fluence limit imaging depth and field of view and can hinder accurate estimation of functional quantities, such as the vascular blood oxygenation. These limitations hinder OAT of large objects, such as a human female breast.
Aim: We aim to develop a measurement-data-driven method for normalization of the optical fluence distribution and to investigate blood vasculature detectability and accuracy for estimating vascular blood oxygenation.
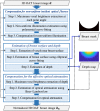
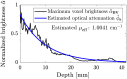
Approach: The proposed method is based on reasonable assumptions regarding breast anatomy and optical properties. The nonuniform incident optical fluence is estimated based on the illumination geometry in the OAT system, and the depth-dependent optical attenuation is approximated using Beer-Lambert law.
Results: Numerical studies demonstrated that the proposed method significantly enhanced blood vessel detectability and improved estimation accuracy of the vascular blood oxygenation from multiwavelength OAT measurements, compared with direct application of spectral linear unmixing without optical fluence compensation. Experimental results showed that the proposed method revealed previously invisible structures in regions deeper than 15 mm and/or near the chest wall.
Conclusions: The proposed method provides a straightforward and computationally inexpensive approximation of wavelength-dependent effective optical attenuation and, thus, enables mitigation of the spectral coloring effect in functional 3D OAT imaging.
Keywords: breast imaging; functional image; optical fluence estimation; optoacoustic tomography; photoacoustic computed tomography; spectral coloring effect.
Figures











References
-
- Oraevsky A. A., et al. , “Laser optoacoustic imaging of breast cancer in vivo,” Proc. SPIE 4256, 6–15 (2001).PSISDG10.1117/12.429300 - DOI
-
- Oraevsky A., et al. , “Full-view 3D imaging system for functional and anatomical screening of the breast,” Proc. SPIE 10494, 104942Y (2018).PSISDG10.1117/12.2318802 - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

