When Poly(A) Binding Proteins Meet Viral Infections, Including SARS-CoV-2
- PMID: 35293770
- PMCID: PMC9006897
- DOI: 10.1128/jvi.00136-22
When Poly(A) Binding Proteins Meet Viral Infections, Including SARS-CoV-2
Erratum in
-
Correction for Gao et al., "When Poly(A) Binding Proteins Meet Viral Infections, Including SARS-CoV-2".J Virol. 2023 Nov 30;97(11):e0098523. doi: 10.1128/jvi.00985-23. Epub 2023 Oct 16. J Virol. 2023. PMID: 37843375 Free PMC article. No abstract available.
Abstract
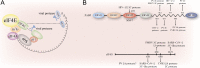
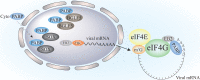
Viruses have evolved diverse strategies to hijack the cellular gene expression system for their replication. The poly(A) binding proteins (PABPs), a family of critical gene expression factors, are viruses' common targets. PABPs act not only as a translation factor but also as a key factor of mRNA metabolism. During viral infections, the activities of PABPs are manipulated by various viruses, subverting the host translation machinery or evading the cellular antiviral defense mechanism. Viruses harness PABPs by modifying their stability, complex formation with other translation initiation factors, or subcellular localization to promote viral mRNAs translation while shutting off or competing with host protein synthesis. For the past decade, many studies have demonstrated the PABPs' roles during viral infection. This review summarizes a comprehensive perspective of PABPs' roles during viral infection and how viruses evade host antiviral defense through the manipulations of PABPs.
Keywords: host defense; poly(A)-binding protein (PABP); viral infection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Pestova TV, Lorsch JR, Hellen CUT. 2007. The mechanism of translation initiation in eukaryotes, p 87–128. In Mathews MB, Sonenberg N, Hershey JWB (ed), Translational control in biology and medicine. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York, NY.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

