SARS-CoV-2 Nsp14 mediates the effects of viral infection on the host cell transcriptome
- PMID: 35293857
- PMCID: PMC9054133
- DOI: 10.7554/eLife.71945
SARS-CoV-2 Nsp14 mediates the effects of viral infection on the host cell transcriptome
Abstract
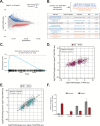
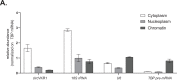
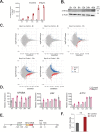
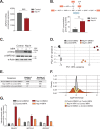
Viral infection involves complex set of events orchestrated by multiple viral proteins. To identify functions of SARS-CoV-2 proteins, we performed transcriptomic analyses of cells expressing individual viral proteins. Expression of Nsp14, a protein involved in viral RNA replication, provoked a dramatic remodeling of the transcriptome that strongly resembled that observed following SARS-CoV-2 infection. Moreover, Nsp14 expression altered the splicing of more than 1000 genes and resulted in a dramatic increase in the number of circRNAs, which are linked to innate immunity. These effects were independent of the Nsp14 exonuclease activity and required the N7-guanine-methyltransferase domain of the protein. Activation of the NFkB pathway and increased expression of CXCL8 occurred early upon Nsp14 expression. We identified IMPDH2, which catalyzes the rate-limiting step of guanine nucleotides biosynthesis, as a key mediator of these effects. Nsp14 expression caused an increase in GTP cellular levels, and the effect of Nsp14 was strongly decreased in the presence of IMPDH2 inhibitors. Together, our data demonstrate an unknown role for Nsp14 with implications for therapy.
Keywords: CXCL8; IMPDH2; Nsp14; SARS-CoV-2; chromosomes; circRNA; gene expression; human; infectious disease; microbiology; transcription.
Plain language summary
Viruses are parasites, relying on the cells they infect to make more of themselves. In doing so they change how an infected cell turns its genes on and off, forcing it to build new virus particles and turning off the immune surveillance that would allow the body to intervene. This is how SARS-CoV-2, the virus that causes COVID, survives with a genome that carries instructions to make just 29 proteins. One of these proteins, known as Nsp14, is involved in both virus reproduction and immune escape. Previous work has shown that it interacts with IMPDH2, the cellular enzyme that controls the production of the building blocks of the genetic code. The impact of this interaction is not clear. To find out more, Zaffagni et al. introduced 26 of the SARS-CoV-2 proteins into human cells one at a time. Nsp14 had the most dramatic effect, dialing around 4,000 genes up or down and changing how the cell interprets over 1,000 genes. Despite being just one protein, it mimicked the genetic changes seen during real SARS-CoV-2 infection. Blocking IMPDH2 partially reversed the effects, which suggests that the interaction of Nsp14 with the enzyme might be responsible for the effects of SARS-CoV-2 on the genes of the cell. Understanding how viral proteins affect cells can explain what happens during infection. This could lead to the discovery of new treatments designed to counteract the effects of the virus. Further work could investigate whether interfering with Nsp14 helps cells to overcome infection.
© 2022, Zaffagni et al.
Conflict of interest statement
MZ, JH, IP, NP, SN, SK No competing interests declared
Figures








Update of
-
SARS-CoV-2 Nsp14 mediates the effects of viral infection on the host cell transcriptome.bioRxiv [Preprint]. 2022 Feb 16:2021.07.02.450964. doi: 10.1101/2021.07.02.450964. bioRxiv. 2022. Update in: Elife. 2022 Mar 16;11:e71945. doi: 10.7554/eLife.71945. PMID: 35194610 Free PMC article. Updated. Preprint.
References
-
- Adcock IM, Hashemian SMR, Mortaz E, Masjedi MR, Folkerts G. Severity of acute respiratory distress syndrome resulting from tuberculosis correlates with bronchoalveolar lavage CXCL-8 expression. International Journal of Mycobacteriology. 2015;4:71. doi: 10.1016/j.ijmyco.2014.11.039. - DOI
-
- Bachmayr-Heyda A, Reiner AT, Auer K, Sukhbaatar N, Aust S, Bachleitner-Hofmann T, Mesteri I, Grunt TW, Zeillinger R, Pils D. Correlation of circular RNA abundance with proliferation – exemplified with colorectal and ovarian cancer, idiopathic lung fibrosis and normal human tissues. Scientific Reports. 2015;5:8057. doi: 10.1038/srep08057. - DOI - PMC - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

