A simple preparation protocol for shipping and storage of tissue sections for laser ablation-inductively coupled plasma-mass spectrometry imaging
- PMID: 35294013
- PMCID: PMC8963325
- DOI: 10.1093/mtomcs/mfac013
A simple preparation protocol for shipping and storage of tissue sections for laser ablation-inductively coupled plasma-mass spectrometry imaging
Abstract
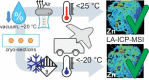
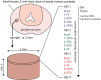
A rapid and cost-efficient tissue preparation protocol for laser ablation-inductively coupled plasma-mass spectrometry imaging (LA-ICP-MSI) has been developed within this study as an alternative to the current gold standard using fresh-frozen samples or other preparation techniques such as formalin fixation (FFix) and formalin-fixed paraffin-embedding (FFPE). Samples were vacuum dried at room temperature (RT) and stored in sealed vacuum containers for storage and shipping between collaborating parties. We compared our new protocol to established methods using prostate tissue sections investigating typical endogenous elements such as zinc, iron, and phosphorous with LA-ICP-MSI. The new protocol yielded comparable imaging results as fresh-frozen sections. FFPE sections were also tested due to the wide use and availability of FFPE tissue. However, the FFPE protocol and the FFix alone led to massive washout of the target elements on the sections tested in this work. Therefore, our new protocol presents an easy and rapid alternative for tissue preservation with comparable results to fresh-frozen sections for LA-ICP-MSI. It overcomes washout risks of commonly used tissue fixation techniques and does not require expensive and potentially unstable and time-critical shipping of frozen material on dry ice. Additionally, this protocol is likely applicable for several bioimaging approaches, as the dry condition may act comparable to other dehydrating fixatives, such as acetone or methanol, preventing degradation while avoiding washout effects.
Keywords: LA–ICP–MSI; human prostate; tissue preparation; zinc.
© The Author(s) 2022. Published by Oxford University Press.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures





References
-
- O'Rourke M. B., Smith C. C., Tse B. C. Y., Sutherland G. T., Crossett B., Padula M. P., `What did i do wrong?' An empirical evaluation of sample preparation methodologies in matrix-assisted laser desorption/ionization-mass spectrometry imaging, Future Sci. OA, 2019, 5(4), FSO362.
-
- Wisztorski M., Franck J., Salzet M., Fournier. In: I., MALDI direct analysis and imaging of frozen versus FFPE tissues: what strategy for which sample?, Methods Mol. Biol., 2010, 656, 303–322. - PubMed
-
- Judd A. M., Gutierrez D. B., Moore J. L., Patterson N. H., Yang J., Romer C. E., Norris J. L., Caprioli R. M., A recommended and verified procedure for in situ tryptic digestion of formalin-fixed paraffin-embedded tissues for analysis by matrix-assisted laser desorption/ionization imaging mass spectrometry, J. Mass Spectrom., 2019, 54(8), 716–727. - PMC - PubMed
-
- Casadonte R., Kriegsmann M., Zweynert F., Friedrich K., Bretton G., Otto M., Deininger S. O., Paape R., Belau E., Suckau D., Aust D., Pilarsky C., Kriegsmann J., Imaging mass spectrometry to discriminate breast from pancreatic cancer metastasis in formalin-fixed paraffin-embedded tissues, Proteomics, 2014, 14(7-8), 956–964. - PubMed
-
- Gustafsson J. O. R., Eddes J. S., Meding S., Koudelka T., Oehler M. K., McColl S. R., Hoffmann P., Internal calibrants allow high accuracy peptide matching between MALDI imaging MS and LC-MS/MS, J. Proteomics, 2012, 75(16), 5093–5105. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

