An artificial host system enables the obligate parasite Cuscuta campestris to grow and reproduce in vitro
- PMID: 35294033
- PMCID: PMC9157073
- DOI: 10.1093/plphys/kiac106
An artificial host system enables the obligate parasite Cuscuta campestris to grow and reproduce in vitro
Abstract
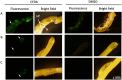
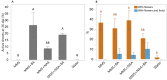
Cuscuta campestris is an obligate parasitic plant that requires a host to complete its life cycle. Parasite-host connections occur via a haustorium, a unique organ that acts as a bridge for the uptake of water, nutrients, and macromolecules. Research on Cuscuta is often complicated by host influences, but comparable systems for growing the parasite in the absence of a host do not exist. We developed an axenic method to grow C. campestris on an artificial host system (AHS). We evaluated the effects of nutrients and phytohormones on parasite haustoria development and growth. Haustorium morphology and gene expression were also characterized. The AHS consists of an inert, fibrous stick that mimics a host stem, wicking water and nutrients to the parasite. It enables C. campestris to exhibit a parasitic habit and develop through all stages of its life cycle, including production of new shoots and viable seeds. The phytohormones 1-naphthaleneacetic acid and 6-benzylaminopurine affect haustoria morphology and increase parasite fresh weight and biomass. Unigene expression in AHS haustoria reflects processes similar to those in haustoria on living host plants. The AHS is a methodological improvement for studying Cuscuta biology by avoiding specific host effects on the parasite and giving researchers full control of the parasite environment.
© American Society of Plant Biologists 2022. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures






References
-
- Alexa A, Rahnenfuhrer J (2021) topGO: Enrichment analysis for gene ontology. R package version 2.46.0. DOI: 10.18129/B9.bioc.topGO
-
- Aloni R (1987) Differentiation of vascular tissues. Annu Rev Plant Physiol 38: 179–204
-
- Bakos A, Fari M, Toldi O, Lados M (1995) Plant regeneration from seedling-derived callus of dodder (Cuscuta trifolii Bab. et Giggs). Plant Sci 109: 95–101 - PubMed
-
- Baldev B (1962) In vitro studies of floral induction on stem apices of Cuscuta reflexa Roxb.-a short-day plant. Ann Bot 26: 173–180
-
- Benvenuti S, Dinelli G, Bonetti A, Catizone P (2005) Germination ecology, emergence and host detection in Cuscuta campestris. Weed Res 45: 270–278
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

