Engineered Production of Strictosidine and Analogues in Yeast
- PMID: 35294193
- PMCID: PMC9171786
- DOI: 10.1021/acssynbio.2c00037
Engineered Production of Strictosidine and Analogues in Yeast
Abstract
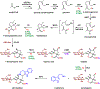
Monoterpene indole alkaloids (MIAs) are an expansive class of plant natural products, many of which have been named on the World Health Organization's List of Essential Medicines. Low production from native plant hosts necessitates a more reliable source of these drugs to meet global demand. Here, we report the development of a yeast-based platform for high-titer production of the universal MIA precursor, strictosidine. Our fed-batch platform produces ∼50 mg/L strictosidine, starting from the commodity chemicals geraniol and tryptamine. The microbially produced strictosidine was purified to homogeneity and characterized by NMR. Additionally, our approach enables the production of halogenated strictosidine analogues through the feeding of modified tryptamines. The MIA platform strain enables rapid access to strictosidine for reconstitution and production of downstream MIA natural products.
Keywords: metabolic engineering; monoterpene indole alkaloids; strictosidine; synthetic biology; yeast.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

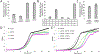
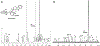
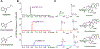
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

