Galectin-1 prevents pathological vascular remodeling in atherosclerosis and abdominal aortic aneurysm
- PMID: 35294231
- PMCID: PMC8926342
- DOI: 10.1126/sciadv.abm7322
Galectin-1 prevents pathological vascular remodeling in atherosclerosis and abdominal aortic aneurysm
Abstract
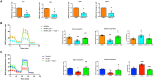
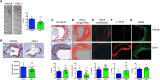
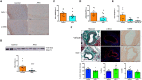
Pathological vascular remodeling is the underlying cause of atherosclerosis and abdominal aortic aneurysm (AAA). Here, we analyzed the role of galectin-1 (Gal-1), a β-galactoside-binding protein, as a therapeutic target for atherosclerosis and AAA. Mice lacking Gal-1 (Lgals1-/-) developed severe atherosclerosis induced by pAAV/D377Y-mPCSK9 adenovirus and displayed higher lipid levels and lower expression of contractile markers of vascular smooth muscle cells (VSMCs) in plaques than wild-type mice. Proteomic analysis of Lgals1-/- aortas showed changes in markers of VSMC phenotypic switch and altered composition of mitochondrial proteins. Mechanistically, Gal-1 silencing resulted in increased foam cell formation and mitochondrial dysfunction in VSMCs, while treatment with recombinant Gal-1 (rGal-1) prevented these effects. Furthermore, rGal-1 treatment attenuated atherosclerosis and elastase-induced AAA, leading to higher contractile VSMCs in aortic tissues. Gal-1 expression decreased in human atheroma and AAA compared to control tissue. Thus, Gal-1-driven circuits emerge as potential therapeutic strategies in atherosclerosis and AAA.
Figures




References
-
- Roth G. A., Mensah G. A., Johnson C. O., Addolorato G., Ammirati E., Baddour L. M., Barengo N. C., Beaton A. Z., Benjamin E. J., Benziger C. P., Bonny A., Brauer M., Brodmann M., Cahill T. J., Carapetis J., Catapano A. L., Chugh S. S., Cooper L. T., Coresh J., Criqui M., DeCleene N., Eagle K. A., Emmons-Bell S., Feigin V. L., Fernández-Solà J., Fowkes G., Gakidou E., Grundy S. M., He F. J., Howard G., Hu F., Inker L., Karthikeyan G., Kassebaum N., Koroshetz W., Lavie C., Lloyd-Jones D., Lu H. S., Mirijello A., Temesgen A. M., Mokdad A., Moran A. E., Muntner P., Narula J., Neal B., Ntsekhe M., Moraes de Oliveira G., Otto C., Owolabi M., Pratt M., Rajagopalan S., Reitsma M., Ribeiro A. L. P., Rigotti N., Rodgers A., Sable C., Shakil S., Sliwa-Hahnle K., Stark B., Sundström J., Timpel P., Tleyjeh I. M., Valgimigli M., Vos T., Whelton P. K., Yacoub M., Zuhlke L., Murray C., Fuster V.; GBD-NHLBI-JACC Global Burden of Cardiovascular Diseases Writing Group , Global burden of cardiovascular diseases and risk factors, 1990–2019: Update From the GBD 2019 study. J. Am. Coll. Cardiol. 76, 2982–3021 (2020). - PMC - PubMed
-
- Libby P., Buring J. E., Badimon L., Hansson G. K., Deanfield J., Bittencourt M. S., Tokgözoğlu L., Lewis E. F., Atherosclerosis. Nat. Rev. Dis. Primers 5, 56 (2019). - PubMed
-
- Bentzon J. F., Otsuka F., Virmani R., Falk E., Mechanisms of plaque formation and rupture. Circ. Res. 114, 1852–1866 (2014). - PubMed
-
- Torres-Fonseca M., Galan M., Martinez-Lopez D., Cañes L., Roldan-Montero R., Alonso J., Reyero-Postigo T., Orriols M., Mendez-Barbero N., Sirvent M., Blanco-Colio L. M., Martínez J., Martin-Ventura J. L., Rodríguez C., Pathophisiology of abdominal aortic aneurysm: Biomarkers and novel therapeutic targets. Clin. Investig. Arterioscler. 31, 166–177 (2019). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

