In vivo prime editing of a metabolic liver disease in mice
- PMID: 35294257
- PMCID: PMC7614134
- DOI: 10.1126/scitranslmed.abl9238
In vivo prime editing of a metabolic liver disease in mice
Abstract
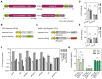
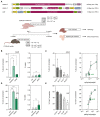
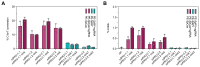
Prime editing is a highly versatile CRISPR-based genome editing technology that works without DNA double-strand break formation. Despite rapid technological advances, in vivo application for the treatment of genetic diseases remains challenging. Here, we developed a size-reduced SpCas9 prime editor (PE) lacking the RNaseH domain (PE2ΔRnH) and an intein-split construct (PE2 p.1153) for adeno-associated virus-mediated delivery into the liver. Editing efficiencies reached 15% at the Dnmt1 locus and were further elevated to 58% by delivering unsplit PE2ΔRnH via human adenoviral vector 5 (AdV). To provide proof of concept for correcting a genetic liver disease, we used the AdV approach for repairing the disease-causing Pahenu2 mutation in a mouse model of phenylketonuria (PKU) via prime editing. Average correction efficiencies of 11.1% (up to 17.4%) in neonates led to therapeutic reduction of blood phenylalanine, without inducing detectable off-target mutations or prolonged liver inflammation. Although the current in vivo prime editing approach for PKU has limitations for clinical application due to the requirement of high vector doses (7 × 1014 vg/kg) and the induction of immune responses to the vector and the PE, further development of the technology may lead to curative therapies for PKU and other genetic liver diseases.
Conflict of interest statement
Figures


References
-
- Ma H, Marti-Gutierrez N, Park SW, Wu J, Lee Y, Suzuki K, Koski A, Ji D, Hayama T, Ahmed R, Darby H, et al. Correction of a pathogenic gene mutation in human embryos. Nature. 2017;548:413–419. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

